Abbreviations
Abs, absorbance; A, hexane extract; B, ethyl acetate extract; C, methanol extract; E, ethanol extract; DMEM, Dulbecco’s modified Eagle’s medium; DMSO, dimethyl sulphoxide; DPPH, 2,2-Diphenyl-2-picrylhydrazyl; IC50, half maximal inhibitory concentration; GC/MS, Gas chromatography-mass spectrometry; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; RT, retention time.
Introduction
TheClusiaceaefamily is characterized by the presence of metabolites with complexity and structural variety and different biological activities. The genus Clusia (Clusiaceae) comprises about 250-300 species [1] and in Cuba, it is one of the genus most represented into of the family, including Garcinia and Calophyllum. For several years, Clusia species have been used in folk medicine to treat various diseases, mainly inflammatory and pain illnesses [2]. The specie C. minor (Quiripití) is traditionally used for the treatment of sores (latex) and warts (leaves) [3], probably due to its anti-inflammatory and antioxidant properties.
Phytochemical studies derived from this genus have revealed the occurrence of polyisoprenylated benzophenones as major metabolites identified [4),(5),(6),(7]. In addition, other metabolites have also been described to be present in the genus, such as flavonoids [4),(8),(9),(10], terpenes [8),(10),(11] and sterols [10),(11]. Some of these compounds and extracts obtained from Clusia species have presented antioxidant [9),(12], anti-inflammatory [13], antibacterial [6),(10] and antitumor activities [8),(14].
Previously, three polyprenylated benzophenones derivatives (named propolone D, hyperibone B and garcinielliptone I) were isolated from C. minor L. fresh fruits [15]. In other way, GC/MS analysis of the hexane extract from its leaves showed the presence of triterpenoids (α, β-amyrins, lupeol, friedelin, epifriedelinol), sterols (β-sitosterol, stigmasterol) and vitamin E as major compounds [16]. Meanwhile, the ethanolic extract analysis led to the identification of sixteen compounds, mainly: triterpenes, steroids and vitamin E. The most commonly compounds found were β-sitosterol (14.04%), α-amyrin (11.94%), vitamin E (8.44%) and β-amyrin (7.82%) (unpublished data).
This study reports the chemical composition of polar extracts (B and C) from C. minor L. leaves by means of Gas Chromatography/Mass Spectrometry. Also, a screening of the pharmaco-toxicological effects of the four extracts obtained from this specie was performed.
Experimental
Chemicals
All reagents and solvents used for gas chromatography/mass spectrometry analysis were analytical quality and obtained from Aldrich (Milwaukee, MN, USA). Culture media and complements were purchased from GIBCO (Gibco BRL, Paisley, UK). The other chemicals were purchased from Sigma Aldrich.
Plant material
Leaves of Clusia minor L. were collected in September 2016 at the National Botanical Garden, Cuba. This specie was identified by Dr. Cristina Panfet and a voucher specimen (Herbarium No. 482) was deposited at the herbarium of the Tropical Agricultural Fundamental Research Institute.
Preparation of the extracts
Dried and grounded leaves (30 g) of C. minor L. were successively extracted with hexane (A), ethyl acetate (B) and methanol (C). Also, 32 g of dried powdered leaves of C. minor L. were successively extracted with ethanol absolute (E). All extracts were obtained by static maceration at room temperature three times during seven days each. Solvents were replaced every two days. Extracts were filtered and concentrated to dryness under reduced pressure at 45 ºC using a rotary vacuum evaporator. Dried extracts were weighed and stored for further analysis. One mg of the dried residue of extracts B and C was dissolved in 1 mL of both ethyl acetate and methanol, and also it was dissolved in 0.5 mL of internal standard solution (tridecanoic acid at 0.05 mg/mL in chloroform). These extracts were analyzed by GC/MS.
GC/MS analysis
For the analysis, the volume of injection was 1 μL without derivatization. The sample was separated on a fused-silica capillary column (SPB-5, 15 m x 0.25 mm i.d., film thickness 0.10 μm, Supelco, PA, USA) installed on a Hewlett-Packard 6890 gas chromatograph (Palo Alto, CA, USA) coupled with a Hewlett-Packard 5973 quadrupole mass spectrometer (GC/MS).
The oven temperature was programmed as follows: isothermic initially at 60 ºC for 2 minutes, then increased up to 100 ºC at 4 ºC/min, then increased up to 290 ºC at 10 ºC/min and finally isothermic for 5 min. The split injection mode (1:10) was used. The carrier gas was helium at a constant flow mode rate of 1 mL/min. The injection port, ion source and interface temperatures were: 280, 280 and 150 ºC, respectively. The energy of ionization was of 70 eV. The mass spectra were obtained in full scan mode since 40 to 700 uma.
The identification of components was accomplished by comparison of retention times (RT) and spectra from available commercial standards (mixtures C8 to C24, α and β-amyrins, β-sitosterol) and similar compound spectra at the libraries NIST98 and Wiley275 MS. Only the components with similarity index of 90% and above according to databases were considered. All compounds were confirmed by molecular ion peaks and the study of classical fragmentation pattern. In some cases where identical spectra were not found, only the structural type of the component was proposed based on the MS fragmentation. The semi-quantification process of major compounds was carried out by internal normalization with the area of each compound. The addition of each area of the compounds corresponds to 100% area.
Assay of 2,2-Diphenyl-2-picrylhydrazyl (DPPH) scavenging activity
The antioxidant capacity of the four extracts was measured as DPPH radical scavenging ability according to Tabart [17] with minor modifications. DPPH (0.075 mg/mL) in ethanol was mixed with the extracts (10-2000 μg/mL) at final volume of 2000 μl. A control sample (absolute ethanol) and a reference sample (absolute ethanol plus DPPH solution) were used. The decrease in the absorbance (Abs) at 515 nm was determined in a UV-1201 spectrophotometer (Shimadzu, Japan), until the reaction plateau step was reached. The IC50 values were determined by using GraphPad Prism program Version 5 and it was defined as the total antioxidant compound necessary to decrease the initial DPPH radical concentration by 50%. Their scavenging effect of each extract was calculated as: % DPPH inhibition = (control Abs - sample Abs)/(control Abs) x 100, were control Abs represent: ethanol Abs + DPPH and sample Abs: sample Abs + DPPH.
MTT assay as a measured of the cytotoxicity effects
Cytotoxic potential was investigated against the non-tumor cells, BHK-21 (baby hamster kidney) and three tumor cell lines: CT26-WT (murine colon cancer cells), 4T1 (mouse metastatic mammary adenocarcinoma) and EAHy926 (human endothelial cells). Cells diluted in Dulbecco’s modified Eagle’s medium (DMEM) with 10 % heat-inactivated fetal calf serum were incubated overnight at 37 oC and 5% CO2. Then, they were exposed to the extracts (10-1000 µg/mL) for 48 h. The extracts were diluted in DMSO (0.5%). Control cells (medium plus DMSO 0.5 %) were included. Cell viability was assessed using a MTT assay as Mossman et al. [18). MTT was added at 1 mg/mL final concentration and the cells were incubated for 3 h. After aspirating the medium, 100 µl of DMSO were added for the dissolution of formazan crystals. The absorbance was read at 540 nm using a microplate reader. The percentage of inhibition of the MTT reduction was expressed as the percentage of viable cells referred to control cells incubated in the absence of test extracts (negative control, it was considered as 100% viability value). The values of IC50 were also calculated.
In vivo assays
Animals
Mice were obtained from Center for the Production of Laboratory Animals (CENPALAB, Havana, Cuba). They were kept in a temperature-controlled environment (23°C) with a 12 h light-dark cycle, relative humidity 40-70%, with food and water ad libitum. OF-1 mice (25-30 g) were randomly assigned for each test. The studies were in compliance with Good Laboratory Practice standards. Experiments were conducted in accordance with ethical guidelines for investigations in laboratory animals and were approved by the Ethical Committee for Animal Experimentation of the Institute of Marine Sciences (ICIMAR), Havana, Cuba.
Croton oil-induced mouse ear edema as a measured of inflammation
Inflammation was induced by topical application of croton oil (2 mg in 20 µL of acetone) of both surfaces of the right ear of each mouse. The left ear of each mouse received the vehicle (control). Each extract was administered topically (0.5-4 mg per ear in acetone) one hour before croton oil. A group with application of croton oil (negative control) on the right ear and a positive control group that received indomethacin (3 mg/20 (L of acetone) were used. The induction of the inflammation lasted five hours and soon after mice were killed by cervical dislocation. A 6 mm section from each ear was removed with a metal punch and weighed. Ear edema was calculated by subtracting the weight of the left ear (vehicle) from the right ear (treatment), and was expressed as edema weight. Inhibition percentage was expressed as a reduction in weight with respect to the control group [19].
Acute oral toxicity study
The study was performed according to the ATC protocol described in the OECD Protocol 423 [20]. Four extracts were administered orally via gastric intubation to mice of both sexes at a single dose of 2000 mg/kg. They were suspended in carboxymethylcellulose 0.5% (10 mL/kg). During experiment, appearance and over behavior were recorded, so changes in skin and fur, eyes and mucous membranes, disturbances on the respiration, circulation, autonomic or central nervous system and behavior pattern were observed. Body weight data was measured at the beginning and the end of the study. The incidence of gross pathological changes observed during the necropsy performed at day 14 was also included.
Statistical analysis
Data were expressed as the mean ( SD. For the GC/MS analyses three independent measures were done on five samples. Statistical analysis was performed by Mann-Whitney U test. The percentage of DPPH scavenged, cell viability and inflammation determinations were analyzed by one-way ANOVA, followed by a Dunnett’s test for multiple comparisons using the GraphPad Prism Version 5 statistical software package. Frequencies of toxicity signs were compared by means of the Fisher exact probability test. A p< 0.05 was considered statistically significant.
Results and discussion
GC/MS analysis of Clusia minor extracts
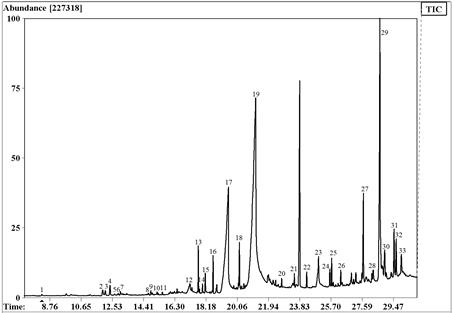
The chromatogram of extract B obtained after the GC/MS analysis showed chromatographic peaks from 8 to 23 min approximately and 33 compounds were identified (Fig. 1, Table 1). Several compounds identified in this extract were also present in the extract A [16], suggesting an elevated abundance of this kind of compounds in the leaves of this specie.
Table 1 Compounds identified in extract B from C. minor leaves by GC/MS.
| Peak No. | Compounds | RT (min) | Relative abundance (% ± SD) |
| 1 | δ-elemene | 8.293 | 0.050 ± 0.009 |
| 2 | selin-4, 7(11)-diene | 11.957 | 0.361 ± 0.015 |
| 3 | β-selinene | 12.116 | 0.285 ± 0.007 |
| 4 | β-maaliene | 12.392 | 0.485 ± 0.022 |
| 5 | α-murolene | 12.669 | 0.072 ± 0.018 |
| 6 | α-aorfene | 12.895 | 0.089 ± 0.054 |
| 7 | 4-hidroxy-3,5-di-tert-butyl toluene | 13.021 | 0.160 ± 0.010 |
| 8 | aromandrene | 14.874 | 0.140 ± 0.012 |
| 9 | cycloisolongifolene | 14.931 | 0.075 ± 0.008 |
| 10 | globulol | 15.234 | 0.097 ± 0.061 |
| 11 | cadalin | 15.544 | 0.122 ± 0.030 |
| 12 | tetradecanoic acid (myristic acid) | 17.212 | 0.945 ± 0.024 |
| 13 | neophytadiene | 17.715 | 1.321 ± 0.005 |
| 14 | 2-hexadecene, 3,7,11,15-tetramethyl | 17.791 | 0.119 ± 0.031 |
| 16 | hexadecanoic acid, methyl ester | 18.604 | 1.012 ± 0.009 |
| 17 | hexadecanoic acid | 19.517 | 19.837 ± 0.005 |
| 18 | 9, 12, 15, octadecatrienoic acid, methyl ester | 20.188 | 1.460 ± 0.060 |
| 20 | tetracosane | 22.736 | 0.243 ± 0.011 |
| 21 | pentacosane | 23.499 | 0.397 ± 0.032 |
| 22 | hexacosane | 24.245 | 0.536 ± 0.0012 |
| 23 | heptacosane | 24.957 | 1.381 ± 0.006 |
| 24 | octacosane | 25.645 | 0.627 ± 0.042 |
| 25 | squalene | 25.754 | 0.874 ± 0.032 |
| 26 | nonacosane | 26.299 | 0.537 ± 0.019 |
| 27 | vitamin E | 27.648 | 3.286 ± 0.010 |
| 28 | stigmasterol | 28.243 | 0.672 ± 0.054 |
| 29 | β-sitosterol | 28.653 | 13.314 ± 0.021 |
| 30 | lupeol | 28.944 | 1.468 ± 0.011 |
| 31 | epifriedelinol | 29.511 | 2.280 ± 0.058 |
| 32 | friedelin | 29.636 | 1.586 ± 0.015 |
| 33 | urs-12-en-28-al | 29.947 | 1.103 ± 0.052 |
Agroup of minority compounds were characterized from 8 to 16 min corresponding mainly to sesquiterpenes (aprox. 2% of total identified compounds). On the other hand, compounds with abundant signal were recorded between 17-30 min corresponding to the majority of compounds (98% of the extract). These substances were identified as straight chain hydrocarbons, pentacyclic triterpenoids derivatives of ursanes, lupanes, and friedelanes presented as alcohol form. Steroids were observed too. Vitamin E represented 3.3% of this extract, while major compounds found were hexadecanoic acid (19.8%) and the steroid β-sitosterol (13.3%).
In this extract, two constituents showed mass spectral fragmentation typical for straight chain hydrocarbons, but their structures could not be determined. They were identified as 15 and 19 in the obtained chromatogram (Fig. 1). Comparisons of mass spectra of 19 and 18 (octadecatrienoate methyl ester) showed several coincidences. The molecular ion of compound 18 was M+ 292 Da, while compound 19 had its molecular ion at m/z 278 Da, 14 Da less than compound 18. Then, compound 19 was proposed as heptadecatrienoate methyl ester, an inferior homologue of octadecatrienoate methyl ester.
Besides, the chromatogram analysis of extract C showed 27 chromatographic peaks with high abundances (Fig. 2, Table 2). As it can be seen, between 23-25 min straight chain hydrocarbon compounds were found corresponding to 49% of the extract. Also, vitamin E was observed in this interval with an abundance of 2.5%. Above 28 min different triterpenoids (lupanes, ursanes, oleananes, friedelanes), tritriacontane and β-sitosterol were found. The most abundant compounds present in this extract were epifriedelinol (23.7%) and friedelin (12.7%).
Table 2 Compounds identified in extract C from C. minor leaves by GC/MS.
| Peak No. | Compounds | RT (min) | Relative abundance (% ± SD) |
| 1 | azulene | 11.848 | 0.364 ± 0.002 |
| 2 | eudesma-4(14),11-diene | 12.007 | 0.332 ± 0.050 |
| 3 | α-selinene | 12.292 | 0.430 ± 0.031 |
| 4 | 4-hydroxy-3,5-di-tert-butyl- toluene | 12.954 | 0.599 ± 0.006 |
| 5 | 1-hexadecene | 18.059 | 1.205 ± 0.015 |
| 6 | hexadecanoic acid | 19.115 | 2.414 ± 0.044 |
| 7 | docosane | 21.026 | 0.502 ± 0.009 |
| 8 | tricosane | 21.856 | 1.020 ± 0.026 |
| 9 | tetracosane | 22.652 | 1.792 ± 0.012 |
| 10 | 2,4-bis (phenylethyl)-phenol | 23.096 | 0.181 ± 0.008 |
| 11 | pentacosane | 23.415 | 3.149 ± 0.030 |
| 12 | hexacosane | 24.153 | 3.566 ± 0.011 |
| 13 | heptacosane | 24.865 | 3.940 ± 0.007 |
| 14 | octacosane | 25.552 | 3.808 ± 0.033 |
| 15 | squalene | 25.653 | 0.947 ± 0.021 |
| 16 | nonacosane | 26.210 | 11.042 ± 0.054 |
| 17 | triacontane | 26.843 | 1.772 ± 0.012 |
| 18 | hentriacontane | 27.480 | 7.928 ± 0.007 |
| 19 | vitamin E | 27.514 | 2.490 ± 0.060 |
| 20 | dotriacontane | 28.076 | 1.596 ± 0.022 |
| 21 | β-sitosterol | 28.436 | 3.308 ± 0.009 |
| 22 | β-amyrin | 28.495 | 2.921 ± 0.012 |
| 23 | tritriacontane | 28.671 | 1.262 ± 0.031 |
| 24 | α-amyrin | 28.746 | 1.741 ± 0.044 |
| 25 | lupeol | 28.788 | 5.187 ± 0.013 |
| 26 | epifriedelinol | 29.375 | 23.770 ± 0.007 |
| 27 | friedelin | 29.492 | 12.722 ± 0.024 |
Based on previous results [16], and according to our collected data nonacosane, squalene, vitamin E, β-sitosterol, lupeol, epifriedelinol and friedelin were found in the three extracts (A, B and C) obtained from this specie of Clusia grown in Cuba.
In the genus Clusia have been identified triterpenoids, such as α and β amyrins [16),(22], friedelin [10),(12),(16, 23], aplotaxene [22], oleanolic acid [22),(24], α and β friedelinols [16),(22),(24]. Meanwhile, we have reported here the presence of α and β-amyrins, friedelin, epifriedelinol and lupeol in Clusia minor L. Lupeol was the most abundant triterpene found in the present study (representing 13.7% of total identified compounds), followed of α-amyrin (10%). These findings confirms the abundance of the triterpenoids in the genus Clusia.
Many of the identified compounds often possess valuable biological activities. Sesquiterpenes have shown antibacterial and antifungal activities, which indicates defensive functions in the plants [21]. Fatty acids and derivatives are known to present antibacterial, anti-inflammatory, anticancer, and antioxidant properties [25),(26),(27]. Triterpene alcohols have been demonstrated to possess marked anti-inflammatory activity [28),(29] and compounds such as α-amyrin, lupeol and cycloartan-type triterpenes are recognized cytotoxic agents [30),(31),(32].
Sterols are important constituents of eukaryotes organisms. They play a vital role in plant cell-membranes. In addition, recently the biological role of phytosterols in human and animal health has been established, with emphasis in its immune modulatory activity [33),(34]. In this study, only one group of sterols (double bonds at C-5) was found after the analysis of extracts B and C. The typical plant sterols, sitosterol and stigmasterol, structurally similar, appeared as major sterol components found. β-sitosterol has been associated with cardiovascular protection, exerting its effect mainly through increasing the antioxidant defense system and effectively lowering the serum cholesterol levels in humans [35]. Only β-sitosterol has been reported found previously in the genus [10),(12),(22] and, stigmasterol was identified for the first time in the genus in the previous study done for this specie [16].
Pharmacological and toxicity screening of Clusia minor extracts
Traditional uses of C. minor L specie involve antioxidant and anti-inflammatory properties [2]. The type of structures identified in the C. minor L. leaves extracts reinforces this evidence so we decided to evaluate their potential in vitro antioxidant effects by determination its scavenging capacity and anti-inflammatory activity in mice.
The data shows that four extracts were able to exhibit DPPH radical scavenging activity. As it shows in Fig. 3, the maximum antioxidant effect (85%) was observed for extract E at 125 µg/mL. In addition, this extract showed the lowest IC50 value (10.25 ± 8.7 µg/mL). As it was previously was explained, extract E was obtained by absolute ethanol extraction, a high polarity solvent. The ethanol favors the extraction of many phenolic compounds like flavonoids, which have been reported to exhibit strong antioxidant activity [36),(37),(38),(39),(40]. As we would have expected, this result matches with compounds identified in this extract by GC-MS analysis (unpublished data). Extracts B and C showed also significant antioxidant activity, being its maximum scavenging capacity of 70 and 80%, respectively, at the concentration of 625 µg/mL while, the IC50 values calculated were 126.2 µg/mL ± 97.6 (B) and 198.9 µg/mL ± 57.3 (C) (Fig. 3). Extract A showed a maximum effect around 58% at 300 µg/mL (IC50 = 132 µg/mL), which was lower than the ones observed for other extracts (B, C and E). These results evidenced that extract polarity may reinforce the capacity to scavenger DPPH. Vitamin E, identified in the extracts, was a common antioxidant and it has strong free radical scavenger capabilities [41]. Other compounds identified in these extracts with recognized antioxidant properties and previously found were stigmasterol [42),(43], β-sitosterol [44),(45),(46], neophytadiene [47].

Fig. 3 Antioxidant activity of the extracts from C. minor L leaves. IC50 values were determined by using GraphPad Prism program Version 5 and it was defined as the total antioxidant compound necessary to decrease the initial DPPH radical concentration by 50%. The scavenging effect of each extract was calculated based on the percentage of DPPH scavenged as: % DPPH inhibition = (control Abs - sample Abs)/(control Abs) x 100, were control Abs represent: ethanol Abs + DPPH and sample Abs: sample Abs + DPPH. Values represent average of three determinations with ± standard deviation. Ascorbic acid was used as standard.
Besides, our results showed C. minor L. leaves extracts only significantly (p < 0.05) attenuated the oil croton-induced inflammation in mice at 4 mg per ear (Table 3), except extract B that was able to significant reduce the inflammation at the dose of 2 mg per ear. These extracts exhibited the highest percentage of the inhibition of the oil croton induced-inflammation showing values in the order of 82%.
Table 3 Effect of C. minor leaves extracts on croton oil-induced oedema in the mouse ear.
| Dose | Extract A | Extract B | Extract C | Extract E | |||||
| Oedema weight (mg) | Inhibition (%) | Oedema weight (mg) | Inhibition (%) | Oedema weight (mg) | Inhibition (%) | Oedema weight (mg) | Inhibition (%) | ||
| Croton oil | 10.8 ± 1.9 | - | 10.8 ± 1.9 | - | 10.8 ± 1.9 | - | 10.8 ± 1.9 | - | |
| Clusia minor extracts | 0.5 | 8.9 ± 4.4 | 17.4 | 9.9 ± 1.2 | 8.3 | 10.6 ± 1.4 | 1.2 | 9.5 ± 1.4 | 11.6 |
| 1 | 6.1 ± 0.4 | 43.9 | 5.9 ± 0.7 | 45.7 | 8.8 ± 1.6 | 18.1 | 7.9 ± 0.8 | 26.7 | |
| 2 | 5.2 ± 1.3 | 51.5 | 5.2 ± 1.6 | 51.7 | 8.4 ± 1.9 | 22.0 | 6.6 ± 1.8 | 38.8 | |
| 4 | 3.1 ± 1.5 | 71.3 | 1.9 ± 0.9 | 82.3 | 6.7 ± 2.4 | 38.3 | 3.4 ± 2.7 | 68.7 | |
| Control + | 3 | 0.6 ± 0.1 | 94.3 | 0.6 ± 0.1 | 94.3 | 0.6 ± 0.1 | 94.3 | 0.6 ± 0.1 | 94.3 |
Each group represents the mean ± S.E.M. of 7-10 animals. Dose, expressed in mg/ear.
Control +: Indomethacin (3 mg/20 (L of acetone), *p≤0.05 statistical significance compared to Croton oil group.
The effects on cell viability were also evaluated in all extracts, and a significant decrease in cell viability was observed after exposure to cell lines during 48 hours to four evaluated extracts. Table 4 shows the IC50, concentration capable of inhibit the 50% of the cell viability, calculated for each extract. In general terms, the data shows that cell viability decreased after the exposure to the extracts from C. minor. The viability of the CT26 colon cancer cells was the least affected. Meanwhile, extract B seems to be the most cytotoxic under our experimental conditions, showing IC50 values of 32.01 for EA.hy926 and 93.42 µg/ml for 4T1 cells. Cell viability was also reduced for BHK cells when exposed to extract B, but the effect was lesser because IC50 was 200.5 µg/ml.
Table 4 Effects of C. minor L. leaves extracts on cell viability.
| Cell line | Extract | Maximum Effect* | IC50 (μg/mL) |
|---|---|---|---|
| BHK | A | 53 | 129.60 |
| B | 60 | 109.3 | |
| C | 50 | 221.2 | |
| E | 56 | 249.3 | |
| 4T1 | A | 56 | 121.9 |
| B | 60 | 93.42 | |
| C | 50 | 101.1 | |
| E | 54 | 124.4 | |
| EA.hy926 | A | 51 | 22.56 |
| B | 54 | 32.01 | |
| C | 41 | 105.5 | |
| E | 52 | 90.99 | |
| CT26 | A | 5 | 353.1 |
| B | 25 | 203.5 | |
| C | 3 | 470.8 | |
| E | 35 | 500.6 |
*Respect to percentage of inhibition of cell viability after 48 h to exposure to the different concentration extracts.
IC50, concentration capable of inhibit the 50% cell viability.
Sesquiterpenes have shown cytotoxic activity against several solid tumor cell lines [21]. Lupeol [30),(31], α and β-amyrins [32], squalene [47], stigmasterol [33),(42], sitosterol [31),(33),(48),(49] have shown cytotoxic activity and capacity to inhibit tumor promotion in cells. Thus, further studies should be achieved to determine which components of this species could be responsible for the cytotoxic activity specifically, and the pharmacological mechanisms involved.
A risk-benefit balance is an important focal point when new candidates are being tested experimentally. In this sense, acute toxicity studies represent the first step for predicting risk-benefit balance of them. Thus, at the end of this characterization, we decided to evaluate acute toxicity potential, with focus on possible neurotoxicity of C. minor extracts. No deaths were obtained after the oral dose administered of the extracts (2 000 mg/kg) in both sexes in mice. However, it was observed some signs of toxicity during the first hours after administration of the extracts, being the most significant ones ptosis and increasing the grooming behavior and reduced explorative activity. They were more pronounced in the animals treated with extracts C and E. The increase of the grooming behavior could be related to a central excitation or sympathetic stimulation. The ptosis, an indicative of a possible sedative action, could be related to a reduced animal alertness due to probably CNS depressant effects of the extracts. But, the observed signs did not lead to the death of any of the animals; this effect appeared immediately after the exposure and the animals recovered their expected behavior between the first 24 hours after the administration. No gross macroscopic alterations were found at necropsy of the animals at the end of the study. Then, the four extracts obtained from C. minor L. leaves were considered as no toxic extracts (unclassified products, according OECD 423 protocol) under our experimental conditions, since a dose of 2 000 mg/kg did not deaths were observed.
As our results show, the extracts from C. minor grown in Cuba are composed of several phytochemical compounds whose diverse bioactive effects have been here documented. Many of the compounds identified in the extracts are known to be pleiotropic, producing multiple effects by acting on several cellular and/or molecular targets. Taking into consideration the important role that oxidative stress and inflammation play in the development of various human diseases, including neurodegeneration and cancer, the activities found by us reasonably sustain the potential use of compounds extracted from C. minor as natural antioxidants with consequent health benefits. However, before final recommendations should be made, it would be necessary to achieve further studies to assess the relevance of such properties on other in vitro and in vivo pharma-toxicological models.
Conclusions
GC/MS analysis of ethyl acetate and methanol extracts of C. minor L. leaves allowed the identification of 33 and 27 compounds, respectively. Hydrocarbons composition was abundant and diverse. The ethyl acetate extract contained carboxylic acids including their derivatives, unsaturated hydrocarbons and the methanol extract were rich in alkanes or paraffin compounds. The abundance of vitamin E in the extracts is of relevant importance due to its pharmacological properties and its current use in modern life. The extracts obtained from C. minor L. grown in Cuba showed antioxidant, cytotoxic and anti-inflammatory properties and no acute toxicity effects in mice, suggesting the potential use of this specie as a new source of medicinal natural products for the prevention and management of human diseases.











 nueva página del texto (beta)
nueva página del texto (beta)