Introduction
Heterocyclic compounds, in particular piperidines, are considered biologically important and are used as vitamins, hormones, and antibiotics [1]. Piperidine nucleus is an important core of many drug molecules. Diverse pharmacological activities of piperidine and its analogs, including antihistamine, anticancer, and antibacterial properties, have been reported [2]. Pyrrolidine and piperidine occupy a unique place in the development of pharmacologically active substances by replacing the nucleus [3),(4]. Piperidine derivatives have been reported to possess significant pharmacological activities such as larvicidal [5], anti-inflammatory [6], local anesthetic [7], anticancer [8], and antimicrobial properties [9].
Thiopyran structures are considered as one of the most important classes of sulfur-containing heterocycles because of their usefulness in accessing certain natural and unnatural products. There has been an increased focus on sulfur-containing heterocyclic compounds because a broad range of biological activities related to the structure have been reported [10]. Thiopyran compounds shows antibacterial [11], anti-hyperplasia [12], anti-psychotic [13], analgesic, and anti-inflammatory [14] activities.
Mosquito larvae can be controlled by insecticides [15),(16),(17] and the best larvicides are natural products and heterocyclic compounds. For instance, N′-tert-butyl-N,N′-dibenzoylhydrazine (RH-5849) was reported as the first nonsteroidal ecdysone agonist in the mid-1980s [18]. The mosquito borne diseases not only cause high levels of morbidity and mortality but also cause great economic loss to health care. Recent estimates from the World Health Organization (WHO) evidenced that malaria accounts for at least 500 million infections and 3 million deaths annually. The prevalence of dengue fever has increased over the last 50 years, and over 2 billion people are under risk in more than 100 countries [19]. In the wake of this, there is an urgent need to develop new insecticides that are environmentally-friendly and biodegradable and can target specific mosquitoes.
Thousands of crops and trees are susceptible, and the disease caused by phytonematodes results in huge agricultural loss annually [20]. Levamisole is used to treat parasitic worm infections [21]. Plants infested by nematodes show retarded growth and development, as well as loss in the quality and quantity of the harvest. Due to environmental problems, nematicides, such as dibromochloropropane (DBCP) and ethylenedibromide (EDB) were withdrawn from the market. Howbeit, some simple coumarins, furocoumarins, and dicoumarolums, display excellent nematicidal activity, and their skeletons have drawn interest for the development of efficient nematicides [15].
Considering these observations, in the present study, we synthesized a series of pyridine-connected piperidine derivatives (2a-g) and 2H-thiopyran (4a-g) derivatives, and screened them for larvicidal, nematicidal, and antimicrobial activities.
Experimental
Materials
All chemicals were purchased from Merck, Sigma-Aldrich and used without further purification. The solvents were dried and distilled prior to use. Merck pre-coated silica gel plates with a fluorescent indicator were used for analytical thin-layer chromatography (TLC). Flash column chromatography was performed using silica gel (Merck). EtOAc-hexane was used as the eluting solvent for TLC and column chromatography. Melting points were recorded in open capillary tubes and were uncorrected. The IR spectra (KBr) were recorded in KBr on a Shimadzu 8201pc (4000-400 cm-1). The ¹H NMR and 13C NMR spectra were recorded on a Bruker DRX-300 MHz. The elemental analysis (C, H, and N) was conducted using an Elementer analyzer model (Varian EL III). The purity of the compounds was checked by TLC with silica gel plates.
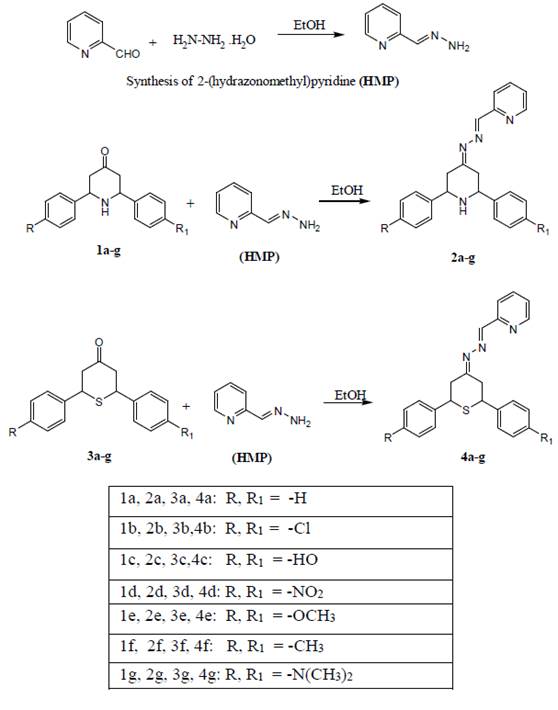
General procedure for synthesis of 2-(hydrazonomethyl)pyridine (HMP)
A mixture of pyridine 2-aldehyde (0.1 mol) and hydrazine hydrate (0.1 mol) in ethanol was heated at refluxed for 5 min. After completed reaction the solid material was filtered and washed with distilled water. The product was confirmed by TLC (hexane-EtOAc, 4:1, v/v). The product (HMP) was purified by flash column chromatography.
General procedure for synthesis of (E)-2-(((2,6-diphenylpiperidin-4-ylidene)hydrazono) methyl)pyridine (2a-g)
A mixture of compound 1a (0.1 mol) and 2-(hydrazonomethyl)pyridine (0.1 mol) in ethanol was heated at refluxed for 2 h. The product 2a was purified by flash column chromatography on silica gel using hexane/EtOAc.
Yellow solid: yield 81%. mp 129-131 ºC; IR (KBr,cm-1): 3045, 3010, 1671, 802, 712; 1H NMR (300 MHz, DMSO-d6): ( 11.15 (1H, s, NH), 8.61 (1H, d, J = 7.4Hz, pyridine), 7.82 (1H, d, J = 7.2Hz, pyridine), 7.71 (1H, dd, J = 7.1, J = 7.0Hz, pyridine), 7.61 (1H, dd, J = 7.1Hz, J = 7.4 Hz, pyridine), 7.59-7.51 (10H, m, aryl), 7.48 (1H, s, -HC=N-), 3.72 (2H, dd, J = 13.7Hz, J = 13.10Hz, 2C-H, 6C-H), 2.51 (2H, dd, J = 13.6Hz, J = 13.04 Hz, 3C-Heq, 5C-Heq), 1.34 (2H, dd, J = 13.4Hz, J = 13.08Hz, 3C-Hax, 5C-Hax); 13C NMR (75MHz, DMSO-d 6 ) (: 168.3 (1C, C=N), 164.2 (1C, C=N), 153.9 (1C), 146.7 (1C), 136.9 (1C), 128.8-127.1 (12C, aryl), 126.1 (1C), 121.0 (1C), 46.8 (2C), 46.1 (1C), 34.5 (1C); EI MS m/z (rel.int): 354 [M]+ (26), 277 (13), 264 (24), 244 (24), 237 (100), 161 (11), 85 (10), 73 (21), 44 (18); Anal C 77.91%, H 6.21%, N 15.80%, Calcd for C23H22N4, C 77.94%, H 6.26%, N 15.81%.
(E)-2-(((2,6-Bis(4-chlorophenyl)piperidin-4-ylidene)hydrazono)methyl)pyridine (2b). Yellow solid: yield 85%; mp 137-139 ºC; IR( KBr, cm-1): 3082, 3020, 1681, 862, 831, 710; ¹H NMR (DMSO-d6): δ 11.23 (1H, s, NH), 8.61 (1H, d, J = 7.4Hz, pyridine), 7.82 (1H, d, J = 7.2Hz, pyridine), 7.71 (1H, dd, J = 7.1, J = 7.0 Hz, pyridine), 7.61 (1H, dd, J = 7.1Hz, J = 7.4 Hz, pyridine), 7.50 (1H, s, -HC=N-) 7.47 (4H, d, J = 7.2Hz, aryl), 7.43-7.39 (2H, d, J = 4.8 Hz, aryl), 7.48-7.44 (2H, d, J = 4.8Hz, aryl), 3.79 (2H, dd, J = 13.7Hz, J = 11.34 Hz, 2C-H, 6C-H), 3.57 (2H, dd, J = 13.4 Hz, J = 11.34 Hz, 3C-Heq, 5C-Heq), 2.89 (2H, dd, J = 11.46Hz, J = 11.46 Hz, 3C-Hax, 5C-Hax); 13C NMR(DMSO-d6): δ 166.1 (1C, C=N), 156.2 (1C, C=N), 152.4 (1C), 146.4 (1C), 136.5 (1C), 126.3 (1C), 121.3 (1C), 128.9 (4C), 128.0 (4C), 140.5 (2C), 131.8 (2C, C-Cl), 61.8 (1C), 54.5 (1C), 46.1 (1C), 36.8 (1C); EIMS m/z (rel.int): 423 [M]+ (26), 346 (14), 333 (18), 318 (23), 306 (32), 237 (100), 161 (16), 85 (28), 73 (19), 44 (10); Anal C 65.46%, H 4.72%, N 13.10%, Calcd for C23H20Cl2N4, C 65.25%, H 4.76%, N 13.01%.
(E)-4,4'-(4-((Pyridin-2-ylmethylene)hydrazono)piperidine-2,6-diyl)diphenol (2c). Yellow solid: yield 80%; mp 145-149 ºC; IR (KBr, cm-1): 3046, 1640, 1451, 828, 711; ¹H NMR (DMSO-d6): δ 11.71(1H, s, NH), 9.56 (2H, s, OH), 8.61 (1H, d, J = 7.4Hz, pyridine), 7.82 (1H, d, J = 7.2Hz, pyridine), 7.71(1H, dd, J = 7.1 Hz, J = 7.0 Hz, pyridine), 7.61 (1H, dd, J = 7.1 Hz, J = 7.4 Hz, pyridine), 7.19-7.10 (4H, d, J = 8.2 Hz, aryl), 6.70-6.67 (4H, d, J = 7.80 Hz, aryl), 7.56 (1H, s, -HC=N-), 3.74 (2H, dd, J = 13.74 Hz, J = 13.10 Hz, 2C-H, 6C-H), 3.32 (2H, dd, J = 13.62 Hz, J = 11.67 Hz, 3C-Heq, 5C-Heq), 2.24 (2H, dd, J = 13.68 Hz, J = 11.39 Hz, 3C-Hax, 5C-Hax); 13C NMR (DMSO-d6): δ 168.7 (1C, C=N), 159.2 (2C, C-OH), 156.4 (1C, C=N), 153.2 (1C), 147.0 (1C), 136.1 (1C), 135.9 (2C), 128.3 (4C), 126.4 (1C), 121.3 (1C), 115.6 (4C), 62.6 (1C), 55.8 (1C), 46.1 (1C), 36.8 (1C); EIMS m/z (rel.int): 386 [M]+ (21), 309 (18), 231 (27), 269 (17), 237 (100), 161 (21), 85 (17), 73 (20), 44 (11); Anal C 71.46%, H 5.77%, N 14.55%, Calcd for C23H22N4O2, C 71.48%, H 5.74%, N14.50%.
(E)-2-(((2,6-Bis(4-nitrophenyl)piperidin-4-ylidene)hydrazono)methyl)pyridine (2d). Yellow solid: yield 74%; mp 155-159 ºC; IR (KBr, cm-1): 3079, 3021, 1684, 1531, 808, 721; ¹H NMR (DMSO-d6): δ 11.28 (1H, s, NH), 8.61 (1H, d, J = 7.4Hz, pyridine), 8.45 (4H, d, J = 7.2 Hz, aryl), 7.82 (1H, d, J = 7.2 Hz, pyridine), 7.72 (1H, s, -HC=N-), 7.71 (1H, dd, J = 7.1 Hz, J = 7.0 Hz, pyridine), 7.61 (1H, dd, J = 7.1 Hz, J = 7.4 Hz, pyridine), 7.29 (4H, d, J = 7.2 Hz, aryl ), 3.79 (2H, dd, J = 13.7 Hz, J = 13.8 Hz, 2C-H, 6C-H), 3.35 (2H, dd, J = 13.8 Hz, J = 11.6 Hz, 3C-Heq, 5C-Heq), 2.11 (2H, d, J = 11.39 Hz, 3C-Hax, 5C-Hax); 13C NMR (DMSO-d6): δ 167.1 (1C, C=N), 157.7 (1C, C=N), 153.7 (1C), 148.4 (2C), 146.4 (1C), 145.2 (2C, C-NO2), 136.1 (1C), 126.7 (1C), 125.9 (4C), 124.8 (4C), 121.5 (1C), 61.8 (1C), 54.5 (1C), 46.1 (1C), 36.8 (1C); EIMS m/z (rel. int): 444 [M]+ (26), 367 (16), 354 (10), 339 (28), 327 (32), 237 (100), 161 (27), 85 (19), 73 (15), 44 (5); Anal C 62.16%, H 4.57%, N 18.92%, Calcd for C23H20N6O4, C 62.16%, H, 4.54%, N 18.91%.
(E)-2-(((2,6-Bis(4-methoxyphenyl)piperidin-4-ylidene)hydrazono)methyl)pyridine (2e). Yellow solid: yield 76%; mp 154-158 ºC; IR (KBr, cm-1): 3085, 3023, 1671, 806, 729; ¹H NMR(DMSO-d6): δ 11.23 (1H, s, NH), 8.61 (1H, d, J = 7.4Hz, pyridine), 7.90 (1H, s, -HC=N-), 7.82 (1H, d, J = 7.2 Hz, pyridine), 7.71 (1H, dd, J = 7.1 Hz, J = 7.0 Hz, pyridine), 7.61 (1H, dd, J = 7.1 Hz, J = 7.4 Hz, pyridine), 7.22-7.20 (4H, d, J = 7.2Hz, aryl), 6.35-6.31 (4H, d, J = 7.2 Hz, aryl), 3.85 (6H, s, -OCH3), 3.72 (2H, dd, J = 13.7 Hz, J = 11.6 Hz, 2C-H, 6C-H), 3.49(2H, dd, J = 13.4 Hz, J = 11.6 Hz, 3C-Heq, 5C-Heq), 2.17 (2H, dd, J = 13.3 Hz, J = 11.4 Hz 3C-Hax, 5C-Hax); 13C-NMR (DMSO-d6): δ 168.2 (1C, C=N), 158.3 (1C, C=N), 157.9 (2C, C-OCH3), 153.3 (1C), 147.2 (1C), 137.8 (1C), 126.2 (1C), 121.5 (1C), 114.8 (4C), 126.9 (4C), 134.7 (2C), 61.8 (1C), 55.1 (2C, OCH3), 54.5 (1C), 46.1 (1C), 36.8 (1C); EIMS m/z(rel.int): 414 [M]+ (26), 336 (21), 310 (19), 297 (34), 237 (100), 161 (10), 85 (10), 73 (18), 44 (6); Anal C 72.46%, H 6.07%, N 13.95%, Calcd for C25H26N4O2 C 72.44%, H 6.32%, N 13.52%.
(E)-2-(((2,6-Di-p-tolylpiperidin-4-ylidene)hydrazono)methyl)pyridine (2f). Yellow solid; yield 81%; mp 167-171 ºC; IR (KBr, cm-1): 3094, 3013, 1651, 825, 710; ¹H NMR (DMSO-d6): δ 11.26 (1H, s, NH), 8.61 (1H, d, J = 7.4 Hz, pyridine), 7.93 (1H, s, HC=N-), 7.82 (1H, d, J = 7.2 Hz, pyridine), 7.71(1H, dd, J= 7.1 Hz, J = 7.0 Hz, pyridine), 7.61 (1H, dd, J = 7.1 Hz, J = 7.4 Hz, pyridine), 7.45-7.40 (4H, d, J = 7.2 Hz, aryl), 7.29-7.26 (4H, d, J = 7.2 Hz, aryl), 3.72 (2H, dd, J = 13.7 Hz, J = 11.6 Hz, 2C-H, 6C-H), 3.36 (2H, dd, J =13.5 Hz, J = 11.6 Hz, 3C-Heq, 5C-Heq), 2.28 (6H, s, CH3), 2.08 (2H, dd, J =13.3 Hz, J = 11.36 Hz, 3C-Hax, 5C-Hax); 13C-NMR(DMSO-d6): δ 167.1 (1C, C=N), 157.6 (1C, C=N), 153.3 (1C), 146.1 (1C), 136.7 (1C), 126.4 (1C), 121.3 (1C), 128.1 (4C), 125.8 (4C), 138.7 (2C), 135.7 (2C, C-CH3), 61.8 (1C), 55.2 (2C, CH3), 54.5 (1C), 46.1(1C), 36.8 (1C)); EIMS m/z (rel.int): 383 [M]+ (31), 305 (27), 278 (19), 265 (09), 237 (100), 161 (08), 85 (16), 73 (16), 44 (5); Anal C 78.46%, H 6.07%, N 14.95%, Calcd for C25H26N4, C 78.50%, H 6.85%, N 14.65%.
(E)-4,4'-(4-((Pyridin-2-ylmethylene)hydrazono)piperidine-2,6-diyl)bis(N,N-dimethylaniline) (2g). Yellow solid: yield 86%; mp 173-175 ºC; IR (KBr, cm-1): 3046, 3010, 1625, 811, 726; ¹H NMR (DMSO-d6): δ 11.28 (1H, s, NH), 8.61 (1H, d, J = 7.4 Hz, pyridine), 7.96 (1H, s, -HC=N-), 7.82 (1H, d, J = 7.2 Hz, pyridine), 7.71(1H, dd, J = 7.1 Hz, J = 7.0 Hz, pyridine), 7.61(1H, dd, J = 7.1 Hz, J = 7.4 Hz, pyridine), 7.19-7.17 (4H, d, J = 7.3 Hz, aryl), 6.40-6.37 (4H, d, J = 7.4 Hz, aryl), 3.69 (2H, dd, J = 13.2 Hz, J = 11.4 Hz, 2C-H, 6C-H), 3.53 (2H, dd, J = 13.1 Hz, J= 11.4 Hz, 3C-Heq, 5C-Heq), 3.14 (12H, s, -N(CH3)2), 2.31 (2H, d, J = 11.22 Hz, 3C-Hax, 5C-Hax); 13C NMR (DMSO-d6): δ 168.6 (1C, C=N), 159.4 (1C, C=N), 153.2 (1C), 146.3 (1C), 136.1 (1C), 126.3 (1C), 121.2 (1C), 149.2 (2C), 112.9 (4C), 131.9 (4C), 133.1 (2C), 60.1 (1C), 54.2 (1C), 46.6 (1C), 40.8 (4C, -N(CH3)2), 37.3 (1C); EIMS m/z (rel.int): 441 [M]+ (32), 363 (28), 351 (20), 336 (19), 323 (7), 237 (100), 161(14); Anal C 73.46%, H 7.07%, N 19.95%, Calcd for C27H32N6, C 73.60%, H 7.32%, N 19.07%.
General procedure for synthesis of (E)-2-(((2,6-Diphenyltetrahydro-4-thiopyran-4-ylidene)hydrazono)methyl)pyridine (4a-4g)
A mixture of compound 3a (0.1 mol) and 2-(hydrazonomethyl)pyridine (0.1 mol) in ethanol was heated at refluxed for 2 h. The product 4a, was purified by flash column chromatography on silica gel using hexane/EtOAc.
Yellow solid: yield 88%; mp 211-214 ºC; IR (KBr, cm-1): 760, 851,3037, 1625, 1752, 3018; ¹H NMR (DMSO-d6): δ 8.61 (1H, d, J = 7.4 Hz, pyridine), 7.82 (1H, d, J = 7.2 Hz, pyridine), 7.78 (1H, s, -HC=N-), 7.71 (1H, dd, J = 7.1 Hz, J = 7.0 Hz, pyridine), 7.61 (1H, dd, J = 7.1 Hz, J = 7.4 Hz, pyridine), 7.45-7.29 (10H, m, aryl), 3.61 (2H, dd, J = 13.73 Hz, J = 11.5 Hz, 2C-H, 6C-H), 3.44 (2H, dd, J = 13.1 Hz, J = 11.5 Hz, 3C-Heq, 5C-Heq), 2.16 (2H, dd, J = 13.1 Hz, J = 11.4 Hz, 3C-Hax, 5C-Hax,); 13C NMR (DMSO-d6): δ 167.9 (1C, C=N), 157.6 (1C, C=N), 153.8 (1C), 146.1 (1C), 142.7-127.0 (12C, aryl), 136.0 (1C), 126.3 (1C), 121.2(1C), 60.7 (1C), 53.6 (1C), 45.9 (1C), 35.7 (1C)); EIMS m/z (rel.int): 371 [M]+ (34), 294 (26), 282 (17), 267 (11), 254 (100), 102 (20), 90 (6), 61(10); Anal C 74.46%, H 5.07%, N 11.95%. Calcd for C23H21N3S, C 74.36%, H 5.70%, N 11.31%.
(E)-2-(((2,6-Bis(4-chlorophenyl)tetrahydro-4H-thiopyran-4-lidene) hydrazono) methyl) pyridine (4b). Yellow solid: yield 82%; mp 245-249 ºC; IR (KBr, cm-1): 3082, 3020, 1742, 1681, 862, 831, 671; ¹H NMR (DMSO-d6): δ 8.61 (1H, d, J = 7.4Hz, pyridine), 7.82 (1H, d, J = 7.2Hz, pyridine), 7.71 (1H, dd, J = 7.1 Hz, J = 7.0 Hz, pyridine), 7.61 (1H, dd, J = 7.1 Hz, J = 7.4 Hz, pyridine), 7.56 (1H, s, -HC=N-), 7.47-7.44 (4H, d, J = 7.3 Hz, aryl), 7.38-7.34 (4H, d, J = 7.3 Hz, aryl), 3.79 (2H, dd, J = 13.74 Hz, J = 11.34 Hz, 2C-H, 6C-H), 3.57 (2H, dd, J = 13.30 Hz, J = 11.34 Hz, 3C-Heq, 5C-Heq), 2.89 (2H, dd, J = 13.34 Hz, J = 11.46 Hz, 3C-Hax, 5C-Hax); 13C NMR (DMSO-d6): δ 166.1 (1C, C=N), 156.2 (1C, C=N), 152.3 (1C), 147.1 (1C), 135.3 (1C), 127.9 (4C), 126.4 (1C), 121.2 (1C), 128.9 (4C), 141.3 (2C), 131.8 (2C, C-Cl), 61.8 (1C), 54.5 (1C), 46.1 (1C), 36.8 (1C); EIMS m/z (rel.int): 440 [M]+ (32), 363 (15), 350 (21), 335 (10), 323 (12), 254 (100), 102 (13), 90 09), 61 (03); Anal C 62.46%, H 4.07%, N 9.95%, Calcd for C23H19Cl2N3S, C 62.73%, H 4.35%; N 9.54%.
(E)-4,4'-(4-((Pyridin-2-ylmethylene)hydrazono)tetrahydro-2H-thiopyran-2,6-diyl)diphenol (4c). Yellow solid: yield 76 %; mp 265-268 ºC; IR (KBr,cm-1): 3046, 1772, 1640, 1451, 828, 614; ¹H NMR (DMSO-d6): δ 9.56 (2H, s, OH), 8.61 (1H, d, J = 7.4 Hz, pyridine), 7.82 (1H, d, J = 7.2Hz, pyridine), 7.71(1H, dd, J = 7.1 Hz, J = 7.0 Hz, pyridine), 7.61 (1H, dd, J = 7.1Hz, J = 7.4 Hz, pyridine), 7.46 (1H -HC=N-), 7.19-7.17 (4H, d, J = 7.3Hz, aryl), 6.70-6.65 (4H, d, J = 7.3 Hz, aryl), 3.74 (2H, dd, J =13.7 Hz, J = 11.6 Hz, 2C-H, 6C-H), 3.32 (2H, dd, J = 13.8 Hz, J = 11.6 Hz, 3C-Heq, 5C-Heq), 2.24 (2H, dd, J =1 3.8 Hz, J = 11.3 Hz, 3C-Hax, 5C-Hax, 1H); 13C NMR (DMSO-d6): δ 168.3 (1C, C=N), 159.2 (2C, C-OH), 156.4 (1C, C=N), 153.0 (1C), 146.2 (1C), 136.7 (1C), 136.3 (2C), 127.9 (4C), 126.4 (1C), 121.1 (1C), 115.6 (4C), 62.6 (1C), 55.8 (1C), 46.1 (1C), 36.8 (1C); EI MS m/z (rel.int): 403 [M]+ (47), 326 (16), 314 (23), 299 (11), 286 (23), 254 (100), 102 (16), 90 (7), 61 (2); Anal C 68.46%, H 5.07%, N 10.95%, Calcd for C23H21N3O2S, C 68.46%, H 5.25%, N 10.41%.
(E)-2-(((2,6-Bis(4-nitrophenyl)tetrahydro-4H-thiopyran-4-ylidene)hydrazono)methyl)pyridine (4d). Yellow solid: yield 86%; mp 255-258 ºC; IR (KBr, cm-1): 3079, 3021, 1711, 1684, 1531, 808, 651; ¹H NMR (DMSO-d6): δ 8.61 (1H, d, J = 7.4 Hz, pyridine), 8.45-8.40 (4H, d, J = 7.8 Hz, aryl), 7.82 (1H, d, J =7.2 Hz, pyridine), 7.71 (1H, dd, J = 7.1 Hz, J = 7.0 Hz, pyridine), 7.61 (1H, dd, J = 7.1 Hz, J = 7.4 Hz, pyridine), 7.40 (1H, s, -HC=N-), 7.29-7.27 (4H, d, J = 7.8 Hz, aryl), 3.79 (2H, dd, J =13.70 Hz, J = 11.67 Hz, 2C-H, 6C-H), 3.35 (2H, dd, J = 13.68 Hz, J = 11.67 Hz, 3C-Heq, 5C-Heq), 2.11 (2H, dd, J = 13.67 Hz, J = 11.39 Hz, 3C-Hax, 5C-Hax); 13C NMR (DMSO-d6): δ 167.1 (1C, C=N), 157.7 (1C, C=N), 153.2 (1C), 148.3 (2C), 147.2 (1C), 145.2 (2C-NO2), 136.1 (1C), 127.0 (1C), 124.9 (4C), 124.6 (4C), 121.3 (1C), 61.8 (1C), 54.5 (1C), 46.1 (1C), 36.8 (1C); EI MS m/z (rel.int): 461 [M]+ (51), 382 (35), 357 (18), 344 (28), 254 (100), 102 (28), 90 (14), 61 (7); Anal C 59.46%, H 5.07%, N 15.95%, S 6.90, Calcd for C23H19N5O4S, C 59.86%, H 4.15%, N 15.18%, S 6.98%.
(E)-2-(((2,6-Bis(4-methoxyphenyl)tetrahydro-4H-thiopyran-4-ylidene)hydrazono)methyl) pyridine (4e). Yellow solid: yield 89%; mp 272-277 ºC; IR (KBr, cm-1): 3085, 1732, 1671, 806, 661; ¹H NMR (DMSO-d6): δ 8.61 (1H, d, J = 7.4 Hz, pyridine), 7.82 (1H, d, J = 7.2 Hz, pyridine), 7.71 (1H, dd, J =7.1 Hz, J = 7.0 Hz, pyridine), 7.61 (1H, dd, J = 7.1 Hz, J = 7.4 Hz, pyridine), 7.22-7.20 (4H, d, J = 7.1 Hz, aryl), 6.35-6.32 (4H, d, J = 7.1 Hz, aryl), 7.43 (1H, s, -HC=N-), 3.85 (6H, s, -OCH3), 3.72 (2H, dd, J = 13.9 Hz, J = 11.6 Hz, 2C-H, 6C-H), 3.49 (2H, d, J = 11.6 Hz, 3C-Heq, 5C-Heq), 2.17 (2H, dd, J = 13.8 Hz, J = 11.4 Hz, 3C-Hax, 5C-Hax); 13C NMR(DMSO-d6): δ 168.2 (1C, C=N), 158.3 (1C, C=N), 157.9 (2C, C-OCH3), 154.2 (1C), 147.0 (1C), 135.8 (2C), 135.2 (1C), 127.1 (1C), 121.4 (1C), 114.3 (4C), 126.4 (4C), 61.8 (1C), 55.8 (2C, -OCH3), 54.5 (1C), 46.1 (1C), 36.8 (1C); EIMS m/z (rel.int): 432 [M]+ (49), 354 (26), 327 (54), 314 (24), 254 (100), 102 (28), 90 (17), 61 (8); Anal C 69.56%, H 5.87%, N 9.75%, S 7.48%, Calcd for C25H25N3O2S, C 69.58%; H, 5.84%; N, 9.74%; S, 7.43%.
(E)-2-(((2,6-Di-p-tolyltetrahydro-4H-thiopyran-4-ylidene)hydrazono)methyl)pyridine (4f). Yellow solid: yield 84%; mp 258-261 ºC; IR (KBr, cm-1): 3094, 1762, 1651, 825, 671; ¹H NMR (DMSO-d6): δ 8.61 (1H, d, J =7 .4 Hz, pyridine), 7.82 (1H, d, J = 7.2 Hz, pyridine), 7.71 (1H, dd, J = 7.1 Hz, J = 7.0 Hz, pyridine), 7.61 (1H, dd, J = 7.1 Hz, J = 7.4 Hz, pyridine), 7.48 (1H, s, -HC=N-), 7.45-7.41 (4H, d, J = 7.4Hz, aryl), 7.29-7.25 (4H, d, J = 7.4 Hz, aryl), 3.72 (2H, dd, J = 13.7 Hz, J = 11.3 Hz, 2C-H, 6C-H), 3.36 (2H, dd, J = 13.5 Hz, J = 11.3 Hz, 3C-Heq, 5C-Heq), 2.28 (6H, s, CH3), 2.08 (2H, dd, J = 13.6 Hz, J = 11.3 Hz, 3C-Hax, 5C-Hax); 13C NMR(DMSO-d6): δ 167.1 (1C, C=N), 157.6 (1C, C=N), 153.1 (1C), 146.0 (1C), 138.7 (2C), 136.1 (1C), 135.7 (2C, Ph-CH3), 128.1 (4C), 126.4 (1C), 125.8 (4C), 121.2 (1C), 61.8 (1C), 54.5 (1C), 46.1 (1C), 36.8 (1C), 21.7 (2C-CH3); EI MS m/z (rel.int): 400 [M]+ 32), 322 (37), 295 (21), 282 (19), 254 (100), 102 (36), 90 (18), 61 (11); Anal C 75.18%, H 6.37%, N 10.55%, S 8.10%, Calcd for C25H25N3S, C 75.15%,; H 6.31%, N 10.52% S 8.03%.
(E)-4,4'-(4-((Pyridin-2-ylmethylene)hydrazono)tetrahydro-2H-thiopyran-2,6-diyl)bis(N,N-dimethylaniline) (4g). Yellow solid: yield 88%; mp 268-270 ºC; IR (KBr, cm-1): 3046, 1782, 1625, 811, 664; ¹H NMR (DMSO-d6): δ 8.61 (1H, d, J = 7.4 Hz, pyridine), 7.82 (1H, d, J = 7.2 Hz, pyridine), 7.71 (1H, dd, J =7 .1 Hz, J = 7.0 Hz, pyridine), 7.61 (1H, dd, J = 7.1 Hz, J = 7.4 Hz, pyridine), 7.46 (1H, s, -HC=N-), 7.23-7.19 (4H, d, J = 7.1Hz, aryl ), 6.48-6.42 (4H, d, J = 7.2 Hz, aryl ), 3.69 (2H, dd, J = 13.2Hz, J = 11.4 Hz, 2C-H, 6C-H), 3.53 (2H, dd, J =13.1 Hz, J = 11.4 Hz, 3C-Heq, 5C-Heq), 3.14 (12H, s, -N(CH3)2), 2.31 (2H, d, J = 11.4 Hz, 3C-Hax, 5C-Hax); 13C NMR (DMSO-d6): δ 168.6 (1C, C=N), 159.4 (1C, C=N), 153.8 (1C), 148.2 (2C, C-N(CH3)2), 146.1 (1C), 136.3 (1C), 133.1 (2C), 131.9 (4C), 126.6 (1C), 121.2 (1C), 112.9 (4C), 60.1 (1C), 54.2 (1C), 46.6 (1C), 40.8 (4C, Ph-N(CH3)2), 37.3 (1C); EIMS m/z (rel.int): 458 [M]+ (16), 381 (43), 354 (24), 341 (17), 254 (100), 102 (21), 90 (10), 61 (3); Anal C 70.46%, H 6.07%, N 15.95%, Calcd for C27H31N5S, C 70.86%, H 6.83%, N 15.30%.
Larvicidal activity
Larvicidal screening was performed following the methodology described in our previous study [22]. Synthesized compounds were tested against the urban mosquito larvae, Culex quinquefasciatus. Eggs of C. quinquefasciatus were obtained from the city drainage system. Eggs were placed in clean water and kept at room temperature for hatching. Larval development was monitored for 7 days. Second stage larvae were collected using a pasture pipette, placed in cotton to remove excess water, and transferred to test vials. Larval mortality was observed using increasing concentrations of synthesized compounds (10, 20, 30, and 40 μg/mL). The susceptibility or resistance of the mosquito larvae to the selected concentration of the synthesized compounds was determined with a standard protocol (WHO, 1981).
Nematicidal activity
Nematicidal activity was evaluated using juvenile nematodes of Meloidogyne javanica [22]. The assay system was prepared with 2 mL Milli-Q® water, containing different concentrations of compound (10, 20, 30, and 40 μg/mL) in glass tubes. Treated and control nematodes were held under the same conditions used for colony maintenance. Ten nematode juveniles were transferred into each tube. Positive (levamisole) and negative (2% DMSO) control tubes were included. Mortality was observed under a zoom stereomicroscope after 24 h of exposure. The mortality percentage was converted into probit scale to determine the LD50 values.
In vitro antimicrobial screening
Antimicrobial activity: The compounds 2a-g, 3a-g, and 4a-g were inspected for their in vitro antibacterial activity against a battery of human type culture pathogens such as Staphylococcus aureus (ATCC-25923), Klebsiella pneumoniae, Escherichia coli (ATCC-25922), and Pseudomonas aeruginosa (ATCC-27853) by disc diffusion method [23] using Mueller-Hinton broth (Hi-media). The same compounds were evaluated for in vitro antifungal activity against a panel of human fungal pathogens such as Aspergillus niger, Candida albicans, Aspergillus fumigatus, Cryptococcus neoformans and Microsporum audouinii using an broth dilution method [24] in Sabouraud’s dextrose broth (Hi-Media).
In order to determine the minimum inhibitory concentration (MIC), tube dilution method was used with respective compounds aliquoted in phosphate-buffered saline (pH 7.2) as test solution. Briefly, the dosing range of compounds were calculated by the factor 2 (anti log 0.3) in order to obtain a dose range between 0.5 to 128 µg per mL in Mueller Hinton broth. Afterwards, respective tubes were inoculated with100 µL of fresh culture of appropriate bacterial and fungal pathogens (104 to 105 CFU/mL) and incubated at 37( 2(C for 24-72 h, respectively. MICs were recorded as the lowest concentrations inhibiting visible growth of the microorganisms as compared to the negative controls.
Statistical analysis
All the experiments were repeated three times to validate the findings statistically [25]. All the data are presented as mean ± standard deviation (S.D.). Mean values were compared among treatments and the control using one way analysis of variance (ANOVA) using SPSS at P< 0.05 levels.
Results and discussion
Chemistry. Compounds 1a-g were synthesized according to the method described previously [26]. Compounds 3a-g were prepared by the method reported elsewhere [27),(28]. Compounds 2a-g and 4a-g were synthesized by condensation method (Scheme 1). The physicochemical data of compounds 2a-g and 4a-g are shown in experimental section. The formation of all the compounds was confirmed by recording the IR, ¹H NMR, 13C NMR spectra, and elemental analyses. The IR spectra of compounds 2a-g showed absorption bands at 3045-3094, and 1625-1684 cm-1 corresponding to the NH and C=N groups, respectively. The ¹H NMR spectra of compounds 2a-g showed a sharp singlet at ( 11.15-11.71 for NH proton and a singlet at ( 7.48-7.96 for HC=N- proton. The 13C NMR spectra of compounds 2a-g showed characteristic peaks at ( 156.2-164.2 and ( 166.1-168.7 ppm corresponding to C=N and -HC=N- carbons, respectively. The IR spectra of compounds 4a-g showed absorption bands at 1711-1782, 1625-1684, and 614-760 cm-1 corresponding to C=N, HC=N, and C-S-C groups, respectively. The ¹H NMR spectra of compounds 4a-g showed signals at ( 7.40-7.78, which confirmed the presence of HC=N- proton. The 13C NMR spectra of compounds 4a-g showed characteristic peaks at ( 166.1-168.6 and ( 156.2-159.4 ppm corresponding to C=N and -HC=N- carbons, respectively. In addition, mass spectra showed that the molecular ion signals matched the expected molecular weights of all the synthesized compounds.
Larvicidal activity. Compounds 2a-g and 4a-g were screened for larvicidal activity. Compounds 2a-g exhibited lower rank of larvicidal activity than compounds 4a-g. Compound 4e showed higher rank of larvicidal activity than other compounds and produced 100% mortality (20 µg/mL), with corresponding LD50 value of 0.8 µg/mL. Compounds 4c, 4d, 4f, and 4g showed moderate activity with concomitant LD50 values of 5.7, 1.2, 8.6, and 6.0 μg/mL, respectively. The values are summarized in Table 1.
Table 1 Larvicidal profile of compounds (2a-g and 4a-g) on second instar larvae of Culex sp.
| Comp.No | Mortality (%)Room temp | LD50 (μg /mL) | |||
| Concentration (μg /mL)a | |||||
| 10 | 20 | 30 | 40 | ||
| 2a | 30.4 ± 1.2 | 64.5 ± 1.5 | 72.3 ± 0.4 | 100 ± 0.0 | 17.4 |
| 2b | 50.3 ± 1.7 | 66.3 ± 1.4 | 82.5 ± 1.0 | 100 ± 0.0 | 10.2 |
| 2c | 39.2 ± 1.3 | 63.9 ± 1.0 | 78.0 ± 0.7 | 100 ± 0.0 | 16.8 |
| 2d | 40.9 ± 1.8 | 61.3 ± 1.2 | 84.7 ± 1.0 | 100 ± 0.0 | 15.7 |
| 2e | 32.2 ± 1.3 | 42.5 ± 1.1 | 69.4 ± 1.2 | 85.6 ± 2.1 | 24.3 |
| 2f | 41.1 ± 0.7 | 51.4 ± 1.0 | 60.5 ± 1.0 | 82.4 ± 1.1 | 19.5 |
| 2g | 32.0 ± 0.6 | 47.4 ± 1.6 | 81.6 ± 0.5 | 100 ± 0.0 | 21.3 |
| 4a | 31.5 ± 0.8 | 62.0 ± 0.5 | 100 ± 0.0 | - | 16.6 |
| 4b | 20.4 ± 0.3 | 40.4 ± 1.0 | 56.8 ± 0.0 | 75.7 ± 0.3 | 26.5 |
| 4c | 62.4 ± 1.3 | 81.5 ± 1.4 | 100± 0.0 | - | 5.7 |
| 4d | 80.3 ± 1.4 | 100± 0.0 | - | - | 1.2 |
| 4e | 88.1 ± 1.9 | 100± 0.0 | - | - | 0.8 |
| 4f | 57.4 ± 0.5 | 84.7 ± 1.0 | 100 ± 0.0 | - | 8.6 |
| 4g | 61.3 ± 1.1 | 78.0 ± 1.1 | 100 ± 0.0 | - | 6.0 |
| Positive control | 43.1 ± 0.3 | 56.7 ± 0.1 | 61.8 ± 1.1 | 100 ± 0.0 | 15.2 |
| Negative control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
a Values are the means of three replicates ± SD.
Positive control: N-tert-butyl-N,N′- dibenzoylhydrazine;
Negative control: Dimethyl sulfoxide (DMSO)
Nematicidal activity. Compounds 2a-g and 4a-g were inspected for nematicidal activity, and compounds 2a-g exhibited lower range of nematicidal activity than compounds 4a-g. The screening was carried out at 37 °C and the toxicity of the compounds were measured. Compound 4e showed higher degree of nematicidal activity than other compounds and produced 100% mortality at 20 µg/mL, with corresponding LD50 value of 3.2 (g/mL. Compounds 4c, 4d, 4f, and 4g showed moderate range of activities with LD50 values of 7.8, 5.5, 4.1, and 4.7 μg/mL, respectively. The values are summarized in Table 2.
Table 2 Nematicidal activity of synthesized compounds (2a-g and 4a-g)
| Comp.No. | Mortality (%)Room temp | LD50 (μg /mL) | |||
| Concentration (μg /mL)a | |||||
| 10 | 20 | 30 | 40 | ||
| 2a | 48.3 ± 3.5 | 62.0 ± 2.9 | 74.4 ± 2.4 | 88.0 ± 0.6 | 11.8 |
| 2b | 40.2 ± 1.6 | 57.6 ± 2.0 | 72.2 ± 1.7 | 85.0 ± 0.0 | 15.5 |
| 2c | 36.1 ± 2.0 | 59.5 ± 3.1 | 64.7 ± 2.0 | 100 ± 0.0 | 17.6 |
| 2d | 48.8 ± 4.4 | 63.0 ± 3.0 | 81.0 ± 3.6 | 100 ± 0.0 | 11.2 |
| 2e | 41.0 ± 3.1 | 59.2 ± 1.6 | 80± 1.0 | 100 ± 0.0 | 17.7 |
| 2f | 48.6 ± 2.0 | 60 .2 ± 3.1 | 100 ± 0.0 | - | 12.5 |
| 2g | 44.5 ± 4.2 | 69.1 ± 1.9 | 81.9 ± 2.9 | 100 ± 0.0 | 20.0 |
| 4a | 54.1 ± 2.1 | 66.7 ± 2.1 | 81.3 ± 1.5 | 100 ± 0.0 | 8.4 |
| 4b | 51.0± 1.9 | 72.6 ± 2.5 | 84.5 ± 1.0 | 100 ± 0.0 | 9.5 |
| 4c | 56.7± 0.3 | 62.0 ± 1.2 | 100± 0.0 | - | 7.8 |
| 4d | 61.3 ± 2.5 | 87.6 ± 1.0 | 100 ± 0.0 | - | 5.5 |
| 4e | 87.8 ± 2.5 | 100 ± 0.0 | - | - | 3.2 |
| 4f | 72.4 ± 2.5 | 83.8 ± 2.5 | 100 ± 0.0 | - | 4.1 |
| 4g | 69.2 ± 2.5 | 86.5 ± 2.5 | 100 ± 0.0 | - | 4.7 |
| Positive control | 40.9 ± 1.1 | 57.8 ± 1.2 | 80.2 ± 1.1 | 100± 0.0 | 14.2 |
| Negative control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
a Values are the means of three replicates ± SD.
Positive control: (-)-Pinidinol;
Negative control: Dimethyl sulfoxide (DMSO)
Antibacterial activity. The synthesized compounds 2a-g, and 4a-g were evaluated for antibacterial activity against various human bacterial pathogens. Compound 2d (MIC: 4 (g/mL) showed high antibacterial activity against E. coli. Compound 2e (MIC: 4 (g/mL) showed higher antibacterial activity against K. pneumoniae than ciprofloxacin (MIC: 16 (g/mL). The MIC values are summarized in Table 3.
Table 3 Antibacterial screening: Minimum inhibitory concentration (MIC) in μg/mL
| Compounds | Gram-positive | Gram-negative | |||
| S. aureus | E. coli | P. aeruginosa | K. pneumoniae | ||
| 2a | 32 | >100 | >100 | 64 | |
| 2b | 32 | 32 | 32 | 32 | |
| 2c | 32 | 32 | 32 | 32 | |
| 2d | 4 | 4 | 8 | 64 | |
| 2e | 8 | 64 | >100 | 4 | |
| 2f | 32 | 32 | 32 | 32 | |
| 2g | 4 | 16 | 16 | 32 | |
| 4a | 64 | >100 | >100 | >100 | |
| 4b | 64 | >100 | >100 | >100 | |
| 4c | 64 | >100 | >100 | >100 | |
| 4d | 64 | >100 | >100 | 64 | |
| 4e | 64 | 64 | 64 | 64 | |
| 4f | 64 | 64 | 64 | 64 | |
| 4g | 64 | 64 | 64 | 64 | |
| Ciprofloxacin | 4 | 8 | 8 | 16 | |
Antifungal activity. Compounds 2a-g and 4a-g were screened for antifungal activity against various fungal pathogens. Compound 4b (MIC: 0.25 μg/mL) exhibited high antifungal activity against C. albicans whereas the compound 4e (MIC: 0.5 μg/mL) showed slight equipotent activity against C. albicans. Compound 4f (MIC: 2 μg/mL) showed higher activity against M. audouinii than clotrimazole (MIC: 4 μg/mL). The antifungal activity values are appended in Table 4.
Table 4 Antifungal screening: Minimum inhibitory concentration (MIC) in μg/mL
| Compounds | A. niger | C. albicans | M. audouinii | C. neoformans |
| 2a | >100 | >100 | >100 | >100 |
| 2b | 32 | 4 | 32 | >100 |
| 2c | >100 | >100 | >100 | >100 |
| 2d | 64 | >100 | >100 | >100 |
| 2e | 64 | >100 | >100 | >100 |
| 2f | 64 | 64 | >100 | >100 |
| 2g | >100 | 64 | >100 | >100 |
| 4a | >100 | 32 | >100 | >100 |
| 4b | >100 | 0.25 | 32 | 32 |
| 4c | 16 | 32 | >100 | 16 |
| 4d | 16 | 8 | 64 | 16 |
| 4e | 16 | 0.5 | >100 | >100 |
| 4f | 32 | 32 | 2 | 64 |
| 4g | 16 | 32 | 16 | 8 |
| Clotrimazole | 1 | 0.5 | 4 | 2 |
Structure-activity relationship
The structure-activity relationship of target compound and the standard is shown in Fig. 1. Compound 4e exhibited high larvicidal and nematicidal activities owing to the presence of pyridine with thiopyran moiety besides CH3O group. The lower degree of larvicidal and nematicidal activities showed by the compounds 2a-g could be due to the presence of piperidine with pyridine rings.
The high antibacterial activity of compound 2e against K. pneumoniae is due to presence of pyridine with piperidine moiety and CH3O group (MIC: 4 μg/mL). The presence of pyridine with piperidine moiety and NO2 group is responsible for the high antibacterial activity of compound 2d against E.coli (MIC: 4 μg/mL). The 4-substituted phenyl ring acts as a lipophilic domain, and the NH group present in piperidine act as a hydrogen bonding domain. It can be suggested that piperidine ring is an essential pharmacophore for antibacterial activity.
The presence of thiopyran moiety with C-l-substituted benzene groups was responsible for the high antifungal activity of compound 4b against C. albicans (MIC: 0.25μg/mL). Compound 4f showed high activity against M. audouinii due to the presence of thiopyran moiety with methyl substituted benzene groups (MIC: 2 μg/mL). Notably, the thiopyran moiety showed significant antifungal activities.
In our previous study [22], the compounds (piperidin-connected 2-thioxoimidazolidin-4-one) were found to be lethal to the second instar larvae of mosquito, which produced a LD50 value of 1.37 μg/mL as compared to pinidinol and hyantocidin (LD50 values of 18.28 and 22.11 μg/mL respectively), however it was low active as compared to compound 4e of present study. Nematicidal activity of previous study [22] showed that the compound (piperidin-connected 2-thioxoimidazolidin-4-one) with LD50 value of 1.57 μg/mL demonstrated high activity as compared to pinidinol and hyantocidin (LD50 values of 14.25, 26.30μg/mL respectively) but very low active in comparison to compound 4e. Therefore, according to the previous study, the 2-thioxoimidazolidin-4-one with piperidin ring showed low potentiality as compared with compound 4e against mosquito larvae and nematodes. At the same time results of the present study evidenced that piperidine series has very low activity as compared to thiopyran 4a-4g series.
Conclusions
Compounds 2a-g and 4a-g were synthesized and screened for larvicidal, nematicidal, and antimicrobial activities. Among the synthesized compounds, 4e showed high larvicidal and nematicidal activities. Compounds 2e and 2d showed high antibacterial activity against K. pneumoniae and E. coli, respectively. Compound 4b exhibited high antifungal activity against C. albicans. Therefore overall results of the present study envisaged that compounds 2e, 2d, 4e, 4b, and 4f can be used as lead molecules for the development of larvicidal, nematicidal, and antimicrobial agents in future.











 nueva página del texto (beta)
nueva página del texto (beta)