Introduction
Enormous amounts of different dyes and pigments are produced for regularly consumption in textile, paper, leather, plastics, food, cosmetics and other industries. Dyes are stable in water and usually resistant to exposure to light and many chemicals. Thus, it is important to find simple and inexpensive procedures for dye removal [1]. MB as a dark green powder is a heterocyclic aromatic chemical that is used widely in biology and chemistry which can cause injuries to humans and animals with symptoms including eyeburns (direct contact), rapid or difficult breathing (inhalation), and also nausa, vomiting, mental confusion and others if ingested [2, 3].
Different chemical and physical treatment methods such as coagulation-flocculation, precipitation, advanced oxidation, ion exchange, membrane filtration, etc have been reported for decolourization of wastewaters. The problem with these methods is the high cost and inadequate performance. Recently, agricultural products and by-products as cheap adsorbents has been widely investigated to introduce instead of current costly methods for removing different types of pollutants (heavy metals ions, hydrocarbons and dyes) from water and wastewaters. Different researchs were done on rice husk [4], peanut husk [5, 6] coffee [7] sawdust [1] banana pith [8], orange peel [9] wheat straw [10], powdered waste sludge [11], wheat shells [12], wheat bran [13] and hen feathers [14]. Modification of agricultural by-product could enhance their natural adsorption capacity [3]. Recently, magnetic separation has been applied in many areas to remove, isolate and/or concentrate the desired components from a sample solution.These technologies are economical, reliable, rapid and durable to treat waste waters by eliminating specific types of pollutants from water [15-19]. Magnetic modification of the low cost adsorbents by magnetic fluids can be leading to formation of magnetic adsorbents which can be easily removed from the treated solutions with magnetic separator.
Peanut husks are one of the biggest food industry waste products. Skin will be separated for processing of peanuts, so a lot of peanut husk is available. Recently, It has been found that peanut husks can efficiently remove copper ions from waste water [20]. So, this study focused on coffee and peanut husk modified with magnetite iron oxide nanoparticles as adsorbents for removal of MB. Peanut in Iran is planted in Golestan, Khuzestan and Guilan provinces. In Guilan province, it is planted mainly in Astaneh Ashrafieh city [21]. Coffee has antioxidant and decrease risk of prostate cancer in men, endometrial cancer in women, liver cancer and diabets and it is useful for hearth. Large amount of coffee produce in the world and most of the people use coffee daily because of its benefites. So the residue of coffee in cafe can be used as a natural adsorbents for elimination of pollution.
The aim of this research is to investigate the usability of magnetic nanoparticles loaded coffee (MNLC) and peanut husk (MNLPH) for MB removal from aqueous solutions.
Materials and methods
Reagents and materials
All used reagents were of analytical reagent grade with the highest purity. MB (Fig. 1) as cationic dye was obtained from Merck (Darmstadt, Germany). Ferric chloride hexahydrate (FeCl3.6H2O), ferrous chloride tetrahydrate (FeCl2.4H2O), hydrochloric acid and perchloric acid were prepared from Merck. Stock solutions of MB were prepared by solving a certain amount of dye in distilled water. These solutions were used for optimization of effective parameters and also for planning calibration curve in order to calculate the dye removal efficiency with UV-V spectrophotometer. All stock and working solutions were prepared using double distilled water.
Instrumentation
A Jenway pHmeter (model 370, England) was used for pH measurements. All of the spectrophotometric measurements of MB in the solutions were done at λmax of MB (660 nm) with a single beam UV-Vis spectrophotometer (Jenway model 6105, England). SEM images, were obtained using digital scanning electron microscope (SEM) (model EM3200, USA). X-ray diffractometer (XRD) (model Xpert MPD, philips, Holland) equipped with a Cu Kɑ radiation source with 2θ range of 0.5-70ᵒ, was used for determining of crystal phases and crystallinity of synthesized materials. The infrared spectra of pretreated peanut husk, Coffee and MNLPH, MNLC were recorded in the wave number range of 400-4000 cm-1 by Bruker Fourier transform infrared spectrophotometer (FT-IR model Alfa, Germany). Coffee Grinder (model MCG 1575) was used for grinding of Peanut husk and Coffee and rotator (model IKA Ms 3 basic, Germany) was used for mixing. For magnetic separation, a strong super magnet (1×3×5 cm) with 1.4 T magnetic field was applied.
Preparation of the adsorbents
Peanut husk was colleted from Astaneh Ashrafieh city.It,s salt was removed by washing with water (three times) and then peanut husk was dried in oven at 100oC for 1 h. The husk was milled in a coffee mill and fraction smaller than 0.5 mm in diameter was collected and used for magnetic modification and coffee were obtained locally.
Preparation of magnetite nanoparticles loaded peanut husk and Coffee
Magnetite loaded peanut husk and coffee were prepared in a similar way as magnetic sawdust. Three grams of powdered husk and five grams of coffee were separately added in a 50 mL polypropylene centrifuge tube containing 40 mL of methanol Then 6 and 5 mL of ferrofluid were added for peanut husk and coffee, respectively. Water-based ionic magnetic fluid stabilized with perchloric acid was prepared using a standard procedure [22]. The suspension was mixed on a rotary mixer (Dynal, Norway) for 1 h. The magnetically modified peanut husk and coffee particles were washed twice with methanol and then air dried [6, 7].
Fig. 2 shows all of the MNLPH and MNLCF adsorbed to magnet and it shows that magnetic nanoparticles loaded natural adsorbents and give them magnetically properties
Result and discussion
Characterization of the MNLC and MNLPH
Characterization of MNLC and MNLPH were studied using XRD and SEM. Fig. 3 shows the SEM images of (a) Coffee, (b) MNLC, (c) pretreated peanut husk and (d) MNLPH. Images indicated that surface of coffee and peanut husk were modified with magnetite nanoparticles in nanometer range and gave it magnetic properties.
The XRD pattern of (a) coffee, (b) MNLC and (c) pretreated peanut husk and (d) MNLPH are shown in Fig. 4. XRD patterns for MNLC and MNLPH include all the coffee, peanut husk and magnetite nanoparticles peaks. The results confirm that Fe3O4 nanoparticles are successfully impregnated onto coffee and peanut husk.The typical peaks of magnetite are observed that can be assigned to magnetite [15]. The results confirm that coffee and peanut husk surfaces were loaded with iron oxide nanoparticles.
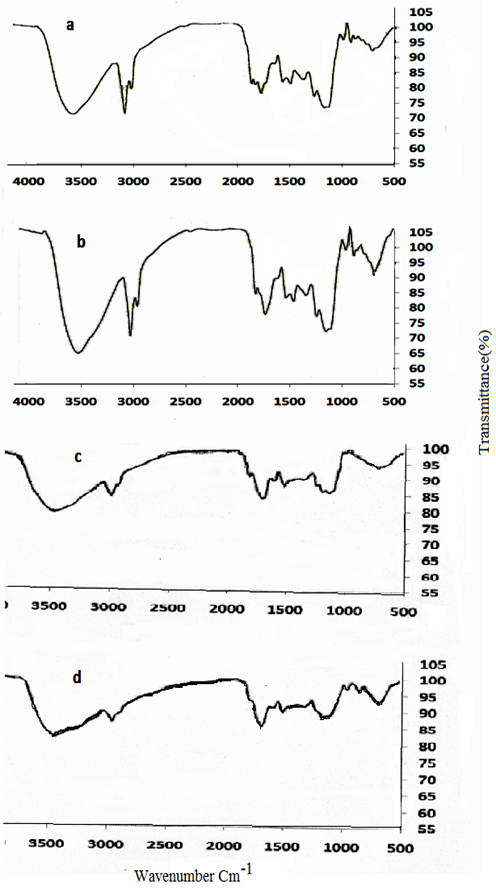
The FT-IR spectra of (a) coffee and (b) MNLC(c) pretreated peanut husk (d) MNLPH are shown in Fig. 5. The spectra display a number of absorption peaks, indicating the complex nature of coffee and peanut husk. The stretching OH groups, are observed in the wave number range of 3200-3550 cm-1 with strong broad band. The band observed at about 2920-2850 cm-1 could be assigned to the aliphatic C-H groups. The trough at 1600 cm-1 represents the benzene ring with C=O. The weaker peak observed at 1378 cm-1 corresponds to the aliphatic nitro compound. According to the Fig. 4 (a to d), the same functional groups were detected on the surface of MNLC and MNLPH. Also, the peak for the Fe-O group was observed at 588 cm-1.
Optimization of dye removal process
In primary experiments the optical absorption behavior of MB at various pHs was studied with measuring absorbance of MB solutions (4 mg L-1) at 660 nm. Various parameters affecting the removal efficiency of MBwere studied and optimized to achieve maximum adsorption efficiency. For optimization studies, 0.03 g of adsorbent (MNLC or MNLPH) was added to 20 mL solution of MB (CMB= 4 mg L-1) in a 50 mL beaker. pH of the solution was adjusted to the desired value using 0.1 mol L-1 HCl and NaOH and the solution was stirred for 30 min. After MB adsorption, adsorbent was quickly separated from the sample solutions using a super magnet (1.4 T). The residual MB concentrations in the supernatant clear solution was determined spectrophotometrically using theproper calibration curve. The following equation was applied to calculate the MB removal efficiency in the treatment experiments:
Dye removal efficiency
Where, Co and Ct are the initial and residual concentrations of the MB.
Effect of amount of adsorbent on the MB removal efficiency
The relationship between adsorption of MB and amount of adsorbent was studied at room temperature and at optimum pH by varying the adsorbent amount from 0.01-0.05 g in contact with 20 mL solution of MB, CMB= 4 and 200 mg L-1 for coffee and peanut husk, respectively. The percentage removal increased by increasing the amount of adsorbents due to the increase in contact surface of adsorbent with MB and the greater availability of the adsorbents with increase the number of adsorption sites availabe for adsorption. Maximum removal of MB reduced with aditional increase in adsorbent dose at constant dye concentration and volume. Particles aggregation saturated adsorption sites and decrease sorption capacity. Aggregation would lead to a decrease in total surface area of the adsorbent and increase in diffusional path length. The adsorption reached a maximum value with 0.03 g (1.5 g L-1) of adsorbents. Therefore, 1.5 g L-1 of adsorbent was selected as the optimum amount for future experiments (Fig. 6).
Effect of contact time on the MB removal efficiency
The effect of contact time on the adsorption of dyes was studied to determine the time taken by adsorbent to remove MB solution CMB= 4 mg L-1 for coffee, CMB= 200 mg L-1 for peanut husk. Absorbance of the residual solution at λmax was measured at different times. It was observed that almost maximum adsorption was occurred after 30 min and this time is enough to reach optimum removal (Fig. 7 (a,b)). Therefore, contact time of 30 min was selected for further works.
Effect of pH on the MB removal efficiency
Solution pH influences adsorption process by affecting both aqueous chemistry and surface binding-sites of the adsorbent. At low pH values, surface of the adsorbent becomes positively charged because of protonation of the functional groups on adsorbent surface. So adsorption of cationic dye decrease because of electrostatic repulsion between dye and protonated adsorbent. As the pH of the dye solution increases, deprotonation of positively charged groups on the adsorbent occur. Electrostatic attraction between negatively charged sites on the adsorbent and dye cations cause increase in adsorption. The effect of pH on the MB removal efficiency was investigated in the pH range of 3.0-12.0 with a stirring time of 30 min. (Fig. 8 (a,b)) shows the removal efficiency of MB as a function of pH. The adsorption efficiency increased by increasing pH and reached maximum at pH=5.0-7.5 for MNLC and pH = 5.0-10.0 for MNLPH.
Effect of ionic strength and speed of mixing on the MB removal efficiency
The effect of ionic strength on the MB adsorption was investigated by addition of NaCl in the range of 0-10% (w/v). The results (Fig. 9) showed that with increase in the NaCl concentration, the Mb removal was reduced for MNLC, whereas it was increased upto 8-10% (w/v) for MNLPH. Therefore, further experiments were done without salt addition.

Fig. 9 Effect of ionic strength on the methylene blue removal.(CMB= 4 mg L-1coffee, CMB= 200 mg L-1 peanut husk, V = 20 mL).
Effect of mixing speed of MB solution and adsorbents on the removal efficiency was studied in the range of 500 to 2500 rpm. The results (Fig. 10) showed that the percent of dye removal was maximum at 1500 rpm due to enhancement in collision between adsorbents and MB dye that increases the adsorption amounts [24]. The rate and speed of MB absorbtion on adsorbent depends on thikness of liquid film surronding adsorbent that is affected by speed of mixing. Percent of removal of MB increased with increase in mixing speed in the range of 500 to 1500 rpm and was maximum at 1500 rpm, but at rates higher than 1500 rpm the removal percent was reduced. It is related to insignificant effect of film thikness in the mixing speed higher than 1500 rpm, hence agitation rate of 1500 rpm was selected for all of the future experiments.
Adsorption mechanism
The most important issue in adsorption research is underestanding mechanism of adsorption. Structure of the adsorbate and adsorbent surface properties should be considered. MB is a cationic dye and in aqueous solution it separates as two ion MB+ and Cl-. An chemical interaction between hydroxyl groups of Fe3O4 magnetic nanoparticles and dye ions occurs.
Based on to the research findings, and due to the structure of the adsorbate and adsorbent surface properties, the adsorption mechanism of MB onto MNLPH and MNLCF may be done according to the following steps:
Movement of MB dye from of the solution to the surface of the adsorbent
Diffusion of MB dye through the boundary layer to the surface of the adsorbent
Adsorption of MB dye on the surface of MNLPH and MNLCF, which may be due to the formation of surface hydrogen bonds between the hydroxyl groups of the magnetic nanoparticles and the nitrogen atoms of MB.
Possible mechanism can be shown as below:
Natural adsorbent- Fe3O4- OH → Natural adsorbent- Fe3O4 - O− + H+
Natural adsorbent- Fe3O4-O− + MB+ → Natural adsorbent- Fe3O4-O-MB + H+
Kinetic and isotherm of adsorption
The study of kinetics of MB adsorption onto MNLC and MNLPH adsorbents is required for selecting optimum operating conditions for the full-scale bath processes. The kinetic parameters, which are helpful for the prediction of the adsorption rate, give important information for designing and modeling the adsorption processes. The kinetic data for adsorption onto MNLC and MNLPH were analyzed using pseudo-first, pseudo-second order, Elovichand intra-particle diffusion models to find out the adsorption rate expression. In the present study, kinetic studies were performed at concentrations of 20 - 100 mg L-1 of MB for MNLC and MNLPH and the solutions were stirred in the time intervals ranged from 0 to 70 min. Then, the clear supernatant solutions were spectrophotometrically measured for residual MB concentrationin the solution. Fig. 11 shows the equilibrium concentrations of MB at the adsorption time interval of 0-70 min. The concentration of residual MB in the solution was monitored and the adsorption capacity qt at time t (qt, mg g-1) was calculated by the following equation:
Where Co and Ct are the initial and equilibrium concentrations of MB at a given time t (mg L-1), respectively. Also,v is the solution volume (mL) and w is the weight of the adsorbent (g).
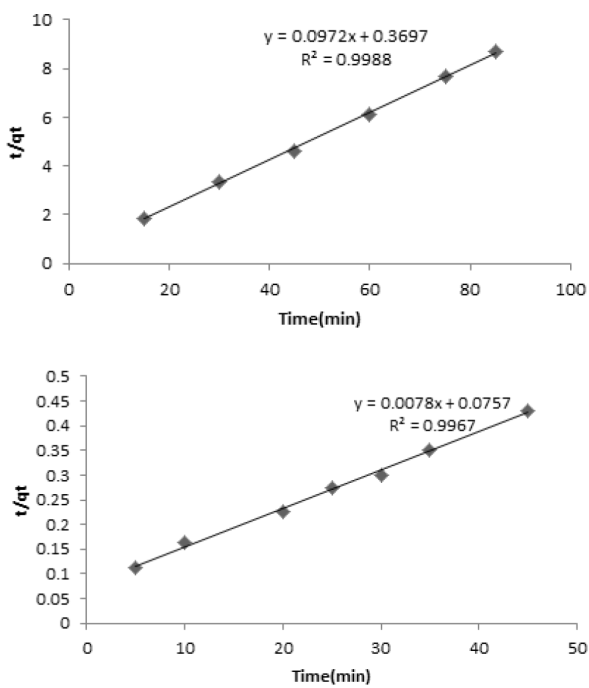
Fig. 11 Fitting of kinetic data to the pseudo-second order kinetic model (CMB=4 mg L-1 for coffee (a) and 200mg L-1 for peanut husk (b) MNLC, MNLPH= 0.03 g, pH=7).
According to the results, the removal rate was very fast during the initial stages of the adsorption process. The kinetic of adsorption was obeyed from pseudo-second order kinetic model according to following equation. The rate of pseudo-second order reaction may be dependenton the amount of solute adsorbed on the surface of adsorbentand the adsorbed amount at equilibrium. The kinetic rateequations for pseudo-second order kinetic model can be written asfollows:
where qt and qe, are the value of adsorbed MB at each time andat equilibrium and k2 (g mg-1 min-1) is the pseudo-second order rate constant. If the second order kinetic equation is applicable, the plot of t/qt against t (Eq(3)) should give a linear relationship. The qe and k2 can be determined from the slope and intercept of the plot. Fitting of kinetic data to pseudo-second order kinetic model, was shown in Fig. 11 (a,b). The best fit of the pseudo-second order kinetic model (R2 close to 1) in the present system shows the adsorption of MB followed by chemisorptions mechanism via electrostatic attraction.
Equilibrium isotherm equations are used to describe the experimental sorption data. The parameters obtained from the different models provide important information on the sorption mechanisms and the surface properties and affinities of the sorbent. The equilibrium adsorption isotherm was determined using batch studies. 20 mL of the MB solution with various initial dye concentrationsin the range of 50-500 mg L-1, was poured into a glass bottle. The time required toreach equilibrium as determined in equilibrium studies was 30 min.The amount of dye uptake by the MNLC and MNLPH, qe (mg g-1),was obtained by Eq. (2).
Adsorption data obtained in a concentration range of 50-500 mg L-1 were correlated with the following linear forms of Langmuir (Eq. (4))[25],
Freundlich (Eq. (5))[26] and Temkin (Eq. (6))[27] adsorption isotherm models:
Langmuir equation:
Freundlich equation:
Tempkin equatin
where qeis the equilibrium concentration of MB onthe adsorbent (mg g-1), Ce is the equilibrium concentration of MB in the solution (mg L-1), qmax is the monolayer capacity of the adsorbent (mg g-1), KL the Langmuir constant (L mg-1) and related to the free energy of adsorption, KF is the Freundlich constant (L g-1) and n (dimensionless) is the heterogeneity factor.
K1 is related to the heat of adsorption (L g-1) and K2 is the dimensionless Tempkin isotherm.
In the Langmuir model, a plot of Ce/ qe versus Ce should indicate astraight line of slope 1/qmax and an intercept of 1/ (KLqmax). The correlation coefficient (R2 Langmuir= 0.949, R2 Freundlich= 0.8993) for Coffee and (R2 Langmuir= 0.9898, R2 Freundlich= 0.4682) for peanut huskshowed strong positive evidence on the adsorption of MB onto adsorbents follows the Langmuir isotherm.This indicates that the adsorption of MB occurs on a homogenous surface by monolayer adsorption without any interaction between adsorbed ions. The value of Qmax for adsorption of MB was obtained from the Langmuir model as 88.49 and 74.62 mg g-1 for MNLC and MNLPH, respectively.
Conclusion
The sorption of pollutants from aqueous solutions plays asignificant role in water pollution control. For this purpose, the utilization of the MNLCand MNLPH as efficient adsorbents wassuccessfully carried out to remove the MB from aquoues samples.The adsorption followed the pseudo-second order kinetic model, suggesting chemisorption. The fit of the Langmuir model in the present system shows the formation of amonolayer covering of the adsorbate at the outer space of the adsorbent. The MNLCand MNLPH are synthesized easily. Due to their very high surface areas, high adsorption capacity can be achieved. The data reported here should be useful for the design and fabrication of an economically treatment process for dye adsorption inindustrial effluents.
Symbols
MNLC: |
magnetite nanoparticles loaded coffee. |
MNLPH: |
magnetite nanoparticles loaded and peanut husk. |
MB: |
methylene blue |
qe: |
Equilibrium dye concentration on the adsorbent. |
Ce: |
Equilibrium dye concentration in the solution. |
qmax: |
monolayer capacity of the adsorbent. |
KL: |
Langmuir constant. |
KF: |
Freundlich constant. |
N: |
Degree of nonlinearity of adsorption. |











 nueva página del texto (beta)
nueva página del texto (beta)