Introduction
Analytical pretreatment is typically carried out by liquid-liquid extraction (LLE) or solid-liquid extraction (SLE) using relatively large volumes of organic solvents. After extraction, the solvent is evaporated from sample solutions, which are then reconstituted using a small volume of solvent compatible with the analytical technique. However, LLE and SLE are time-consuming and involve several steps. Additionally, both techniques suffer from environmental and safety issues because of the toxic and flammable nature of the typical solvents utilized for extraction.
Material science has played a crucial role in overcoming issues related to analytical sample preparation. Particles and coatings have been synthesized, characterized and used as sorbents in environmentally friendly sample preparation techniques such as solid-phase extraction (SPE), matrix-solid phase extraction (MSPD), solid-phase micro extraction (SPME) and stir-bar sorptive extraction (SBSE). Applications of these techniques have been reported [1] to isolate and preconcentrate analytes in environmental, food, medical and pharmaceutical analysis.
In particular, SBSE is a sorption-based technique for the extraction and preconcentration of organic compounds from aqueous samples prior to chromatographic analysis [2]. SBSE enables a great capacity for preconcentration, requires a relatively low sample volume, and can operate overnight without any special requirements. In SBSE, a stir bar coated with poly(dimethylsiloxane) (PDMS) is introduced into the aqueous sample. After stirring the sample for a certain time, the analytes are extracted by the PDMS coating. Then, the stir bar is removed from the sample, and the analytes are released by thermal (TD) or liquid desorption (LD) [3].
Due to the non-polar character of commercial PDMS coatings, SBSE applications have only focused on the extraction of non-polar to weakly polar compounds and failed for the extraction of polar compounds [3]. Thus, PDMS/ethylene glycol stir bars were introduced into the market for the extraction of hydrophilic analytes; however, there is a lack of effective applications for such stir bars [2]. Therefore, only a small number of SBSE coatings are available to extract a large assortment of analytes present in samples with a diverse matrix composition. To overcome this drawback of SBSE, new polymeric coatings have been developed using sol-gel technology.
The sol-gel technology involves a hydrolysis and condensation reaction, which are catalyzed by an acid (or base) [4-7]. The reactions occur simultaneously at room temperature and enable the synthesis of organic or hybrid organic-inorganic polymers. Analytical applications of the sol-gel mainly result from chemical binding of the synthesized polymer to the exposed hydroxyl groups on the surface of the support (e.g., glass). This feature provides mechanical, thermal, and chemical stability to the polymer. These properties are important in the experimental operation of SBSE, such as stirring, thermal and liquid desorption [8].
Another advantage of the sol-gel is the wide variety of commercially available organoalkoxysilanes [R’4-x -Si(OR)x], where R denotes an organic functional group. This enables the free choice of compounds to modify the adsorption properties of the coating, facilitating the preparation of tailor-made coatings for SBSE. Thus, chemical groups, such as β-cyclodextrin (PDMS/β-CD), divinylbenzene (PDMS/DVB), poly(vinyl alcohol (PDMS/PVA), cyanopropyltriethoxysilane, and amino-modified carbon nanotubes, have been introduced into the PDMS network by sol-gel technology [9]. The modified PDMS coatings were tested by extraction of organophosphorous pesticides, organic sulfur compounds, non-steroidal anti-inflammatory drugs, brominated flame retardants, steroid hormones, and phenols [9].
In this paper, a novel material for SBSE was obtained by harnessing advances in material science for use in analytical chemistry. We propose a sol-gel method to obtain PDMS modified by phenyl groups. The modified PDMS was characterized by its water contact angle, infrared spectroscopy, and scanning electron microscopy. Additionally, a thermogravimetric analysis (TGA) was carried out to assess the temperature tolerated by the coating under TD conditions. The stability under LD conditions was evaluated by submerging the coating into different organic solvents and aqueous solutions at different pH values. The potential application of the coated stir bars for SBSE was evaluated using benzene derivatives, antibiotics, and furanic compounds. These compounds are associated with environmental and toxicological concerns and can exist in foods, beverages, and ecosystems [10-12].
Experimental
Chemicals
Hydroxylated poly(dimethylsiloxane) (PDMS-OH, ~550 Mn), triethoxyphenylsilane (TEPS), 5-hydroxymethylfurfural (5-HMF), benzene (BEN), naphtalene (NAP), fluorene (FL), anthracene (ANT), 5-methylfurfural (5-MFA), furfural (2-FAL), 2-furyl methyl ketone (2-FMC), methyl 2-furoate (M2F), sulfathiazole (STZ), sulfametoxazole (SMX), trimetoprim (TMP), chloranphenicol (CRP), and 2,2,4-trimethylpentane (isooctane) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Formic acid (HCOOH), trifluoroacetic acid (TFA), methanol (MeOH), acetone (Ac), sodium hydroxide (NaOH), hydrochloric acid (HCl), methylene chloride (DCM), nitric acid (HNO3), ethyl acetate (EtOAc), and acetonitrile (ACN) were purchased from J.T Baker (Phillipsburg, NJ, USA) and used as received.
Sol-gel synthesis
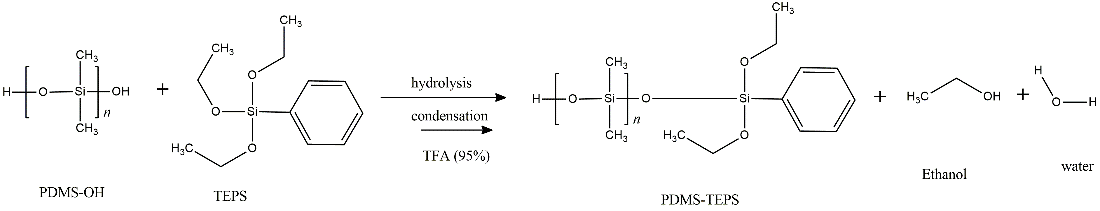
The molar ratio of PDMS-OH:TEPS was 1:3. The sol-gel solution was prepared by adding PDMS-OH (210.5 µL) and DCM (300 µL) into a polypropylene flask before being vortexed (30 s, maximum speed). Then, TEPS (263.2 µL) was added into the flask and vortexed (1 min). Next, 50 µL of a TFA aqueous solution (95 v/v %) was added. Finally, the sol-gel solution was vortexed (2 min, maximum speed), sonicated (5 min), and aged at room temperature in a desiccator (25°C, 12 h).
Coating and stir bar preparation
Glass microscope slides (7 x 23 mm) and a glass capillary tube (12 x 1.5 mm) with a stainless-steel bar encapsulated inside were soaked (3 h) in NaOH (1 M) to activate surface silanol groups. Both were rinsed with distilled water and dipped (3 h) into HCl (0.1 M). Finally, both were washed with distilled water and dried in a convection oven (125 °C, 2 h) [13-15]
Treated glass slides were dipped (2 h) into the sol-gel solution. Then, they were dried for 2 h at 60 °C on a hot plate. The coating procedure was repeated three times with a freshly prepared solution to obtain a multilayer coating. The glass slides were cured in a vacuum oven set at 40 °C, 60 °C, 80 °C and 100 °C; each temperature was hold for 24 h. Finally, the samples were cured at 180 °C for 4 h.
The dried glass slides were taken out of the oven and left to cool at ambient temperature. Two-thirds of each glass slide was coated to leave one end free to manipulate. The glass substrate was carefully coated by avoiding contact with any surface during the process. Once the coating and curing process were completed, the resulting materials were weighed and stored in propylene bags until further use.
A polyvinyl chloride mold was used to fabricate the coated stir bar for SBSE. The sol-gel solution was deposited into the mold containing the previously treated glass capillary tube. After polymerization, the bar was removed from the mold and cured in a vacuum oven set to the same temperature program used for the glass slides. The final dimensions of the stir bars were 12 mm (length) and 1.9 ± 0.05 mm (thickness).
Thermogravimetric analysis
The thermal stability of the hybrid polymer coating was evaluated using a thermogravimetric analyzer, Netzsch STA 449 F3 Jupiter Model (Selb, Germany), under a N2 atmosphere. The scans for thermal decomposition were taken between 25 and 900 °C at a heating rate of 10 °C·min-1.
Contact angle
The wettability of the coating was evaluated by measuring the contact angle (θc). This was measured at 25 ºC by a camera (Moticam10) and Motic Images Plus 2.0 software. The sessile drop method was used to measure the contact angle of de-ionized water (20 µL) on the coated glass slide at 2 different spots. Ten substrates were measured to obtain an average value of θ and its standard deviation.
Organic solvents and pH stability
The solvent stability tests were realized with solvents typically used in SBSE (water, MeOH, ACN, isooctane, Ac, or EtOAc). The tests were carried out by weight loss measurements on a Sartorius Model 54 analytical supermicrobalance. First, the coated glasses were weighed. Then, they were immersed in MeOH and an ultrasonic bath for 10 min. Next, the coated glasses were left to stand in the hood (30 min) before being dried in a convection oven (125 °C, 3 h). Then, the coated glasses were immersed in 1 mL of solvent at room temperature and placed into an ultrasonic bath (15 and 45 min) or vortex (30 and 60 min, maximum speed). Finally, the samples were removed from the liquids and placed onto tissue paper before being weighed. The average weight difference was recorded (n = 3).
The coated glasses were weighed and then immersed (48 h) into aqueous solutions at different pH values (1, 3, 6, 9, and 12). Acid solutions were prepared with HCl or HNO3; basic solutions were prepared with NaOH. The coated glasses were removed after 48 h, wiped with blotting paper and weighed. The average weight difference was recorded (n = 3).
Surface changes on the polymeric coatings due to the solvent and pH stability tests were observed by a MOTIC® stereoscopic microscope and SEM.
SBSE experiments
SBSE experiments were carried out using coated, homemade stir bars. Benzene derivatives, furanic compounds, and antibiotics (STZ, SMX, TMP, and CRP) were all used as probe analytes to evaluate the sorption performance of the coated stir bars. In the experiments, the coated stir bars were immersed into an aqueous solution spiked with analytes (10 mL, 1 mg/L). The extraction (time, agitation, and effect of NaCl and ACN) and LD parameters (solvent, time) were evaluated for each group of probe analytes. The concentration (mg/L) of each analyte in the extracts was determined by HPLC. The area of the chromatographic peak was used for quantification process.
Chromatographic analysis
The extracts were analyzed with a liquid chromatograph (Agilent Technologies Model 1100) (Santa Clara, CA, USA) equipped with a diode array detector, degasser, and quaternary pump. The instrumental parameters used to separate and detect each group of analytes in the SBSE extracts are given in Table 1.
Table 1 HPLC parameters used to analyze the probe compounds in the extracts obtained by SBSE and LD.
| Column | C18 column (5 µm, 4.6 x 150 mm) | ||
| Injection volume | 20 µL | ||
| Flow rate | 1 mL/min | ||
| Temperature | Ambient, 25°C | ||
|
Mobile phase gradient |
Furanic derivatives 0 min 95 % B, 5 min 77 % B 7 min 77 % B 10 min 95 % B |
Benzene derivatives 0 min 40 % B 15 min 80 % B 17 min 80 % B 22 min 40 % B |
Antibiotics 0 min 20% B 10 min 50% B 13 min 50% B 15 min 20% B. |
| Solvents | A: ACN B: Water |
A: ACN B: Water |
A: HCOOH (0.1% v/v)/ MeOH (9/1 v/v, pH 4.5) B: MeOH 0.1% HCOOH |
|
Detection wavelength |
278 nm (5-HMF, 5-MFA, 2-FAL, 2-FMC) 248 nm (M2F) |
254 nm | 278 nm |
Results and Discussion
Sol-gel synthesis
Fig. 1 shows the scheme for the synthesis of acid-catalyzed sol-gel silica coatings by the sol-gel polymerization of TEPS and PDMS-OH. At low pH levels, the silica tended to form occasionally cross-linked linear molecules. These entangled molecular chains probably formed additional branches, resulting in gelation [16]. Following the sol-gel transition, the solvent phase was removed from the network by vacuum drying, yielding a dense coating [17].
The cured coating deposited onto the glass slide was transparent with a homogeneous, pinhole-free surface (Fig. 2). The transparency or refractive index depends on the RSiO3/2, which can be controlled by changing the organic functional group and the temperature used in the curing process. Catagiri [18] increased the transparency of thick films by applying a heat treatment at approximately 400 °C following the electrophoretic sol-gel deposition of PhSiO3/2 particles. However, the PDMS-TEPS coating used here was transparent before and after the curing process.
Scanning electron microscopy
The SEM micrograph (Fig. 3) suggests that the optical transmittance (Fig. 2) is due to the lack of open spaces and cracks that can otherwise cause light scattering. The morphological transformation of the films should cause an increase in the transparency and a decrease in the thickness of the films. This indicates an adequate sol-gel synthesis and a suitable curing process, which enables the formation of a dense film similar to that reported in the literature [15].
Table 2 shows the experimental weight percentage of the elements in the PDMS-TEPS obtained by SEM-EDS as well as that calculated from the reaction stoichiometry. The calculated C/Si ratio (2.2) was higher than the experimental ratio (1.21). This suggests that the TEPS is well-distributed with PDMS-OH in the sol-gel solution which leaves on the surface a low availability of phenylsiloxanes that can be detected by EDS [19].
FTIR analysis
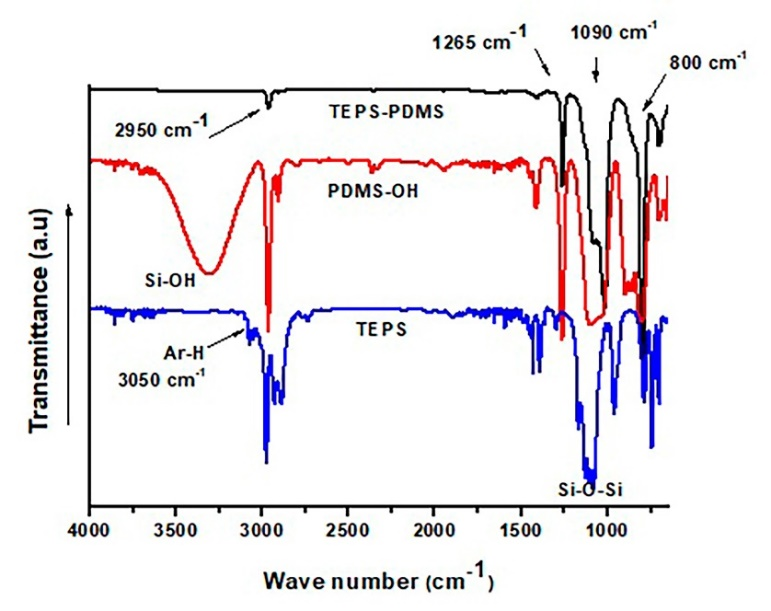
Fig. 4 shows the FTIR spectra of TEPS, PDMS-OH, and the hybrid coating PDMS-TEPS. All spectra showed peaks at 1090 cm-1 and 800 cm-1, which are associated with the asymmetric stretching and bending of the Si-O-Si linkage, respectively. These two peaks in the PDMS-TEPS spectrum confirm the incorporation of a TEPS and PDMS structure inside the coating. The absence of an absorption band at approximately 3000 cm-1 for PDMS-TEPS implied complete hydrolysis and condensation of the silanol groups during the hybrid coating synthesis. The methyl stretching band (C-H) in PDMS appeared at 2950 cm-1. This wavenumber is shorter than that for the C-H absorption of the precursors due to the hydrolysis and condensation reactions that occur in the sol-gel synthesis [15,20,21].
Thermogravimetric analysis
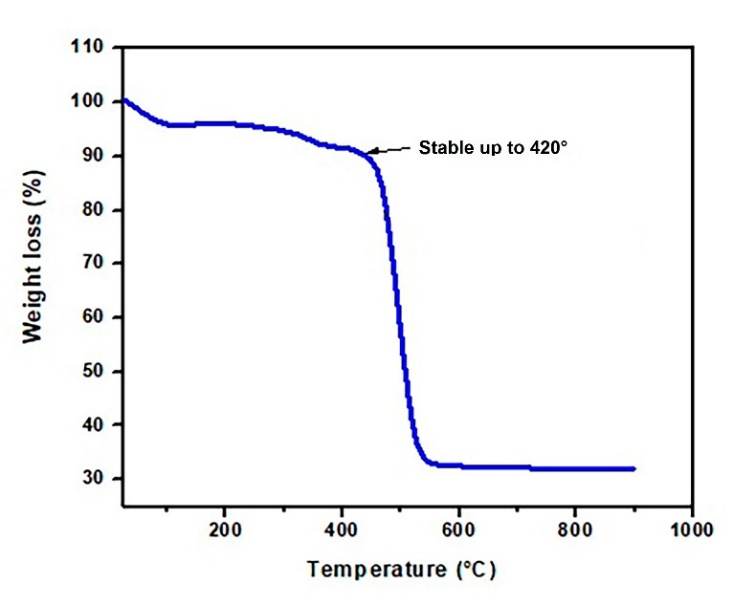
The thermal decomposition curve (Fig. 5) shows that the hybrid coating was stable up to 420 °C (weight loss 10%), which was previously reported for crosslinked PDMS [22]. The material decomposes at 490°C with a weight loss of 60%, which is attributed to the degradation of the organic material in the coating. Above 600 °C, 30% of the material remains, which can be ascribed to the inorganic fraction. This percentage agrees with the ratio of the organic/inorganic materials expected in the crosslinked PDMS coating [22]. This result suggests that the temperature can be used to desorb analytes from stir bars coated with PDMS-TEPS. However, temperatures below 400 °C should be used in any thermal desorption unit to minimize damage to the coating.
Water contact angle
The contact angle is a superficial property that predicts the hydrophilicity or hydrophobicity of a solid material. PDMS has previously shown poor wettability by water with a contact angle close to 100° [23,24]. In our study, the PDMS-TEPS network decreases the water contact angle to 76° ± 3°, which implies that the addition of TEPS leads to a more hydrophilic character. Therefore, it is possible that TEPS improves the interaction between the materials and polar compounds.
Organic solvents and pH stability
The solvent stability is an important parameter for coatings intended to be used as a sorbent in SBSE. The coating should not be soluble in the matrix sample or in the solvent used for LD. The resistance of the hybrid PDMS-TEPS coating was tested by exposure to organic solvents with different polarities using a vortex (45 min) or ultrasonic bath (60 min). In both cases, none of the tested solvents produced a weight loss greater than 1%.
The PDMS-TEPS hybrid coating was immersed into aqueous solutions with different pH values. The weight loss was measured to be lower than 0.5%, which indicated that the coating was very stable for the pH range tested (2-12). Therefore, by setting the pH of the water samples with an appropriate buffer, the coating can be used to extract ionizable compounds by SBSE.
Application of stir bars coated with PDMS-TEPS for SBSE and liquid desorption
The precursor TEPS has been introduced in the PDMS network to promote π- π interactions [25[. Therefore, the PDMS-TEPS coating was tested for stir bar sorptive extraction of aromatic hydrocarbons, furanic derivatives, and antibiotics which contain π-electrons in their chemical structure. Different experimental conditions were evaluated for the extraction (time, agitation, and the effect of NaCl and ACN) and LD steps (solvent, time) to obtain the best analytical signals for each group of analytes.
NAP, ANT, and FL showed a high affinity for the PDMS-TEPS coating. High concentrations were obtained in the extract following SBSE without the need for an organic modifier or salting out (Table 3). This behavior has been reported when PDMS-TEPS was applied as coating to extract PAHs from water with in an array capillary in-tube solid-phase microextraction cartridge [25]. Considering the level of spiking (1 mg/L), a pre-concentration factor varying from six to eleven-fold was achieved for the compounds tested (Fig. 1S - Electronic Supplementary Material). An exception was BEN, which is more volatile than its derivatives and can be more easily lost during the SBSE experiment.
Table 3 Concentration of benzene and its derivatives in SBSE extract obtained from aqueous solutions using a stir-bar coated with PDMS-TEPS.
| Extraction conditions* | Desorption conditions | Concentration (mg/L) | |||
|---|---|---|---|---|---|
| BEN | NAP | FL | ANT | ||
| 1000 rpm, 120 min | 500 µL ACN:H2O (4:1) UB 10 min |
2.20 | 9.65 | 10.88 | 6.41 |
| 1000 rpm, 120 min, 2 % ACN | 2.21 | 10.09 | 11.69 | 8.64 | |
| 1000 rpm, 120 min, 20 % ACN | 0.88 | 6.67 | 8.73 | 9.10 | |
| 1000 rpm, 120 min, 2 % NaCl | 1.39 | 9.08 | 10.11 | 4.88 | |
| 1000 rpm, 120 min, 30 % NaCl | 1.50 | 7.05 | 3.84 | 0.79 | |
n = 3;
*1 mg/L of each analyte in aqueous sample;
UB - Ultrasonic bath
The addition of an organic modifier (ACN 2% v/v) slightly improved the concentrations in the extract and enabled pre-concentration factors close to twelve-fold. However, an increment in ACN (20 % v/v) compromised the concentrations of BEN, NAP, and FL.
Salting out with NaCl at 2% led to concentrations similar to those obtained without it. In contrast, an increment of salt at 30% led to decreased concentration of NAP, FL and ANT. This result can be ascribed to the high density of the aqueous solution, which limits the diffusion of the analytes into the coating.
The PDMS-TEPS coating was tested for stir bar sorptive extraction of benzene and derivatives from sea water and well water both spiked at 1 mg/L. The HPLC chromatograms (Fig. 2S) shown that the coating can extract and preconcentrate the analytes in presence of matrix samples without the addition of an organic modifier or salt. However, the high concentration of salt in sea water decreased the extraction of NAP, FL and ANT as it was observed in model solutions with 30% of NaCl (Table 2). In summary, the PDMS-TEPS coating can be applied to extract other polyaromatic hydrocarbons or compounds with similar polarities from environmental water samples.
The affinity of the PDMS-TEPS coating for furanic compounds and antibiotics was evaluated (Fig. 3S and Fig. 4S). The phenyl groups attached to the PDMS turn the coating into a material that is relatively more hydrophilic (contact angle test). Consequently, an enhanced affinity for polar compounds through π- π, dipole-dipole, or π-dipole interactions was expected due to the presence of π-electrons in the target molecules. However, the concentration of both the probe analytes after SBSE were low (Tables 4 and 5) even with the addition of NaCl. A systematic study must be carried out to better assess the suitability of the PDMS-TEPS coating for the extraction of furanic compounds and antibiotics from aqueous solutions.
Table 4 Concentration of furanic compounds in SBSE extract obtained from aqueous and organic solutions using a stir-bar coated with PDMS-TEPS
| Medium* | Extraction conditions |
Desorption conditions |
Concentration (mg/L) | ||||
|---|---|---|---|---|---|---|---|
| 5-HMF | 2-FAL | 2-FMC | 5-MFA | M2F | |||
| Aqueous | 100 rpm 180 min |
500 µL MeOH UB 45 min |
ND | 0.16 | 0.17 | 0.22 | 0.86 |
| 100 rpm 180 min 30 % NaCl |
ND | 0.20 | 0.26 | 0.35 | 1.19 | ||
| Isooctane | 100 rpm 180 min |
1.48 | 0.20 | 0.14 | 0.20 | 0.14 | |
n = 3;
*1 mg/L of each analyte in aqueous and organic sample;
UB -Ultrasonic bath
Table 5 Concentration of probe antibiotics in SBSE extract obtained from aqueous and organic solutions using stir bars coated with PDMS-TEPS
| Medium* | Extraction conditions |
Desorption conditions |
Concentration (mg/L) | |||
|---|---|---|---|---|---|---|
| STZ | TMP | SMX | CRP | |||
| Aqueous | 750 rpm 120 min pH 4.5 15% NaCl |
500 µL 40% MeOH- 60% HCOOH pH 4.5, UB 40 min |
0.09 | 0.03 | 0.14 | 0.11 |
| Isooctane | 250 rpm 120 min |
2.4 | 2.3 | 2.7 | 1.95 | |
n = 3;
*1 mg/L of each analyte in aqueous and organic sample;
UB - Ultrasonic bath
An additional experiment was carried out to test the affinity of furanic compounds and antibiotics for the PDMS-TEPS coating. The target analytes were dissolved in isooctane (non-polar solvent) to observe their affinity for the siloxane backbone and phenyl moieties. The concentration of most of the furanics were low, but the most polar furanic (5-HMF) was preconcentrated and detected (Table 4, Fig. 3S). This result is in stark contrast to the extraction in aqueous solutions; i.e., 5-HMF was not detected, and the concentration of M2F was higher than that of the other compounds. Additionally, the concentration for the antibiotics improved from 17 to 77-fold when the extractions were carried out in isooctane (Table 5, Fig. 4S).
It is possible that isooctane diffused into the coating and caused swelling, which facilitated the interaction of the analytes with the siloxane chains and phenyl moieties. However, the desorption step can also reduce the concentration of polar analytes. Thus, further studies should be carried out to optimize the overall performance of the SBSE process.
The results for the extraction of furanic compounds and antibiotics from aqueous and organic media suggest that the PDMS-TEPS coating has the potential to be used in SBSE. Despite the low extraction achieved from aqueous media, the hybrid coating can be used to clean-up lipophilic molecules for an analysis focused on furanic derivatives, antibiotics, or other polar molecules. Additionally, the extraction of furanics and antibiotics from isooctane implies that the PDMS-TEPS coating can be used to sample polar molecules released in lipophilic media. For example, the thermolytic breakdown of the cellulose present in paper-based insulators used in electrical transformers leads to the release of furanic compounds and other polar molecules into transformer oil [26].
Conclusions
A translucent coating based on PDMS-OH and phenyltriethoxysilane was synthesized by a sol-gel method. The coating was characterized by SEM and FTIR, which showed the formation of a network structure with a homogeneous, crack-free morphology. The contact angle measurements showed that the coating was relatively polar. The coating was thermally stable up to 400 °C, demonstrating its potential compatibility with TD. Additionally, the coating was stable when immersed in organic solvents with different polarities and aqueous solutions with pH values ranging from 1 to 12. Therefore, the coating is suitable for the extraction of ionizable compounds from water, which can be subsequently released by LD using neat solvents or mixtures (water/MeOH or water/ACN).
The coatings were useful for the extraction and preconcentration of benzene, naphthalene, anthracene, and flourene from aqueous solutions. The PDMS-TEPS coating was able to extract and preconcentrate naphthalene, fluorene and anthracene from well water and sea water. Nevertheless, the matrix of sea water decreased the extraction of analytes.
In addition, the coating was used for stir bar sorptive extraction of five furanic derivatives and four antibiotics from aqueous and organic solutions (isooctane). The extraction of furanic derivatives and antibiotics from water was limited. However, 5-HMF and antibiotics were successfully extracted from isooctane. Therefore, the coating can be used to extract polar compounds from an organic medium.











 nueva página del texto (beta)
nueva página del texto (beta)