Introduction
This review covers the role of analytical chemistry in environmental geochemistry studies conducted across Mexico. To this aim it is first important to define what we understand by environmental geochemistry as well as which aspects of the field will be covered in this paper. Environmental geochemistry is commonly defined as the study of the sources, and distribution of potentially toxic elements (PTEs) in the surface environment, including their speciation and distribution pathways in water, soils, sediments, plants, animals, and humans. Environmental geochemistry may also involve the effects of various elements on human health, although this aspect, to the author’s view, corresponds primarily with toxicological or medical geology studies. Although the fate of organic contaminants in water, soils and sediments may also be classified under environmental geochemistry studies, this theme will not be considered in this review.
It is clear from the above that research in this area cannot be conducted without analytical chemistry. The role of analytical chemistry in environmental geochemical studies may be divided into two primary aspects: (1) qualitative and quantitative determinations of the PTEs (mainly heavy metals) and typical geogenic contaminants (such as As and F-), in total or in their chemical species, and (2) the use of analytical chemistry tools to understand the geochemical behavior of the elements in the aforementioned environmental compartments. A review of both aspects within the studies that have been developed in Mexico, mainly over the past 15 years will be provided in this paper. The objectives of the studies, the analytical methods the studies used, the authors’ interpretations of their results, the areas each study examined, and the substrates (water, soils, and sediments) the studies analyzed will all be summarized in the paper. Although many studies have been developed on the distribution and impact of PTEs in plants and animals in Mexico, this aspect will not be covered in detail in this paper.
Studies may be generally classified as having three main objectives: (1) to determine the concentrations of PTEs in different matrices (2) to identify the sources of the pollutants, and (3) to evaluate the environmental geochemical processes of the elements that were analyzed. The first objective also includes a comparison of the measured concentrations with national and international environmental standards or guidelines, the determination of spatial distribution and/or temporal variations, and a determination of the environmental hazard or risk of PTEs based on indices or risk levels.
Reported environmental geochemistry studies that involve analytical chemistry have focused on soils, groundwater, superficial water bodies, water off coastal areas, and marine, fluvial, and lake sediments. In addition to determining concentrations of PTEs, studies on sediments and soils have included other analytical determinations such as mineralogy, concentrations of the elements naturally present in these substrates in high concentrations, such as K, Si, Al, Fe, and Na; compounds that may affect the geochemical behavior of PTEs as organic matter; and various physico-chemical parameters such as pH, conductivity and particle size. This paper will primarily include the analytical methods applied specifically to determine the concentrations of the PTEs that each study examined, as well as their mineralogy. Due to the extensive literature that exists on this topic, this review will be constrained to studies on sediments, soils, and groundwater, that have been published in international journals over the past 15 years (i.e., since 2003).
The concentrations measured in the studied areas have been compared with diverse environmental quality indices and international and national guidelines or standards including the effects range low (ERL) and effects range medium (ERM), the sediment quality guidelines known as probable effect level (PEL) and threshold effect level (TEL), the quality criteria known as low effect level (LEL) and severe effect level (SEL), and Screening Quick Reference Tables (SQuiRT’s) concentrations. A summary of these indices and limits is presented below.
The guideline values ERL and ERM establish the concentration ranges in order to estimate the adverse biological effects of a chemical present in sediments. Concentrations below the ERL correspond to rarely observed effects, values between the ERL and ERM correspond to possible effects, and concentrations equal to or above ERM correspond to probable adverse effects [1]. With sediment quality guidelines, sediment’s concentrations that correspond to TEL are those where biological effects are rarely observed in benthic organisms; effects are frequently observed in those that correspond to PEL [2]. The quality criteria [3] that reports values that correspond to LEL and SEL allow for classifying those sediments with concentrations above the SEL as highly contaminated that are toxic for aquatic life. The SQuiRT provides screening concentrations for inorganic and organic contaminants in various environmental compartments associated with different probabilities of adverse biological effects [4].
Many investigations of sediments and soils also consider enrichment factors (EF) [5]. The value of the EF is calculated by obtaining the ratio of the measured element with respect to a selected conservative element (most commonly Al, Li, or Fe in Mexican studies), both in the sample and in the background:
where EF is the enrichment factor, Cn(sample) and Cref(sample) are the measured concentrations of the studied element and the reference element in the sample (respectively), and Bn(baseline) and Bref(baseline) correspond to the concentrations of the studied and reference elements in the baseline, respectively. Enrichment factors values are also used to assess the degree of contamination. Ranges are defined as: EF < 2, deficiency to minimal enrichment; EF 2-5, moderate enrichment; EF 5-20, significant enrichment; EF 20-40, very high enrichment; and EF > 40, extremely high enrichment [6]. The geoaccumulation index [7] is also frequently calculated to evaluate the pollution degree of sediments and soils. The geoaccumulation index is defined as:
where Igeo corresponds to the accumulation index, Cn is the measured concentration of the element in the samples, and Bn corresponds to the element’s background concentration. The results are then compared with the Muller index which has seven classes [8] as shown in Table 1.
Table 1 Geoaccumulation index (Igeo) values corresponding to a pollution degree
| Class | Igeo value | Pollution degree |
|---|---|---|
| 0 | ≤ 0 | Unpolluted |
| 1 | >0-1 | Unpolluted to moderately polluted |
| 2 | >1-2 | Moderately polluted |
| 3 | >2-3 | Moderately to strongly polluted |
| 4 | >3-4 | Strongly polluted |
| 5 | >4-5 | Strongly to very strongly polluted |
| 6 | >5 | Very strongly polluted |
Marine Sediments
Many studies have been conducted on marine sediments collected along the Mexican coastline. For example, Frías-Espiricueta et al [9] determined concentrations of heavy metals (Cd, Co, Cu, Fe, Ni, Pb and Zn) in surface sediments of the Huizache-Caimanero Lagoon of northwestern Mexico within the Gulf of California; the authors used analysis of variance to determine concentration differences among the sampling sites, and performed chemical analyses using atomic absorption spectrometry (AAS). Rosales-Hoz et al [10] evaluated the spatial distribution and temporal variations of heavy metals in sediments collected from the Gulf of Mexico, surrounding the coral Isla de Sacrificios which is offshore of Veracruz City; the heavy metals’ potential source and environmental risk were also asessed. Heavy metals were analyzed using flame and graphite furnace (GF) AAS and major elements were analyzed using X-ray fluorescence (XRF).
Determinations of the geochemical fractionation of metals and metalloids in sediments have been used in many studies to evaluate their environmental mobility and possible sources. This methodology consists of the treatment of the solid matrix with various extractants, either sequentially or in different aliquots which then releases the elements retained in different compounds and with different strengths within the solid. Although various methods are applied to this end, the method proposed by Tessier et al [11], and the more recent BCR (Community Bureau of Reference) method [12,13] are the most common.
One example of the application of sequential extraction in sediments is found in Ochoa-Valenzuela et al [14]. In addition to a determination of the spatial distribution of Al, Cd, Cr, Cu, Fe, Mn, and Pb in sediments collected from Bacochibampo Bay in the northwestern state of Sonora, the authors also assessed these elements’ geochemical fractionation and possible sources (through the determination of EF). The heavy metals’ toxicity was evaluated by comparing their values with the LEL values. Chemical analysis was performed by AAS with flame and GF.
Ruiz-Fernández et al [15] assessed historical metal contamination adjacent to the coast of the Coatzacolacos River in Veracruz State. Changes in sediment transport and PTE concentrations were determined by examining a 210Pb-dated sediment core; the results were then used to assess pollution sources. The threats of As, Hg, and Ni were estimated by comparing these elements’ concentrations with US Environmental Protection Agency (EPA) ERL values. Major element and PTE´s concentrations were determined by XRF, and clay mineralogy was characterized by X-ray diffraction (XRD). Isotopic analyses which also included 226Ra, 137Cs, and 13C, were conducted via alpha-spectrometry, gamma-ray spectrometry, and infrared mass spectrometry.
The spatial distribution of Hg and 210Pb was evaluated in sediments of the Estero de Urías coastal lagoon southeastern Gulf of California by Raygoza-Viera et al [16]. Statistical treatment of the concentrations was used in conjunction with organic matter, particle size, carbonates, and reference-elements distribution to explain the spatial variability in Hg and 210Pb concentrations. The reference elements were analyzed by flame and GF-AAS, and Hg by AAS with cold vapor; concentrations of 210Pb were determined through the analysis of 210Po via alpha-spectrometry.
Various environmental geochemistry investigations have been developed along the coastline of Baja California. Arsenic concentrations in marine and stream sediments showed the environmental impact of historical gold mining in San Juan de los Planes and La Ventana Bay in southeastern Baja California. The results indicated a potential risk to biota and humans alike based on ERL and ERM concentrations. Arsenic was analyzed by hydride generation (HG) AAS in this study [17]. Another study on the sediments found on the Baja California Peninsula conducted by Shumilin et al [18] evaluated the environmental impact of the port of Santa Rosalía on the peninsula. The spatial distribution of Cd, Co, Cu, Mn, Pb, U, and Zn (analyzed by inductively coupled plasma mass spectrometry [ICP-MS]) was determined at 13 stations; the enrichment factors showed values above 1 for each of those elements. The results when compared with sediment guidelines, revealed Cu and Zn concentrations to be the most dangerous for marine biota.
Pérez-Tribouillier et al [19] determined the concentrations of more than 50 trace elements in the sediments of La Paz Lagoon by ICP-MS and ICP-AES. The interpretations included the calculation of enrichment factors (with Li as the normalizing element) and statistical principal component analysis (PCA) to ascertain associations among elements. As, Cd and U were found to originate from phosphorites; a comparison with sediment quality indices did not show a probable impact on the biota.
Huerta-Diaz et al [20] evaluated the spatial distribution of trace metal (Al, Cd, Co, Cu, Mn, Fe, Ni, Pb, and Zn) contamination and concentrations in sediments at Ensenada and El Sauzal harbors in Baja California; analyses were performed by AAS. Enrichment factors in which Al was considered as conservative element indicated an anthropogenic enrichment of Pb and Zn. Shumilin et al [21] characterized the spatial distribution of As (measured by instrumental neutron activation [INAA]) in the sediments of the Magdalena-Almejas lagoon complex, while Gutiérrez-Galindo et al [22,23] determined Hg (analyzed by cold vapor-AAS), Ag, and Cd (analyzed by flame and GF-AAS) distribution in surficial sediments from Todos Santos Bay. Grain size and organic carbon were found to control the distribution of Ag and Cd. In one study [24] conducted at Bahía Concepción, the influence of geothermal sources on the concentrations of As and Hg in sediments (determined by HG and cold vapor AAS) was determined. The results showed a rapid decrease in sediments from an adjacent mangrove lagoon.
Jara-Marini and García-Rico [25] studied sediments collected from four sites at the Sonoroan coastal zone to determine As (measured by HG-AAS) distribution in three geochemical fractions: exchangeable, bound to organic matter, and residual. The results showed that As is primarily present in the residual fraction, which implies a low level of risk to biota. Méndez et al [26], however, found moderate to high levels of contamination (estimated with the geoaccumulation index) of Cu, Zn, and Pb, and very strong pollution by Cd in sediments at several sites within the Sonora’s Guaymas Bay. In addition, Green-Ruiz at al. [27] found moderate to strong contamination of Hg (measured by cold vapor AAS) via ER and geochemical index values of sediments collected in the same bay.
South of Sonora, in Sinaloa, Jara-Marini et al [28] reported the spatial distribution of the total and operational bio-available fractions of Cd, Cu, Pb, and Zn (measured by AAS) in sediments collected at Mazatlán harbor. An increase of total concentrations for Cu and Zn was observed regarding to those measured from 1983 to 1994, while Pb and Cd were found to have decreased during the same period; bio-available fractions were also found to have increased from 1983 to 1994. The processes and potential sources of the heavy metals were evaluated using statistical methods and EF calculations.
Sediments in Lakes, Lagoons and Dams
Various studies have assessed the historical accumulation of PTEs in sediments through the analysis of sediment cores collected in lakes and dams. This chronological trend has been studied in Zempoala and Moramar Lagoons, Lake Pátzcuaro, and the Intermedia and Silva dams using 210Pb and 137Cs determinations (via gamma-spectrometry) for dating. Concentrations of metals and metalloids (via ICP-MS and cold vapor AAS) have been compared among the lakes and evaluated regarding quality guidelines for freshwater ecosystems. Possible sources both natural (such as El Chichonal’s volcanic eruption) and anthropogenic (such as the decreased use of leaded gasoline) have been identified to influence PTE concentration variations [29]. At the Culiacán River Estuary in Sinaloa, concentrations of Cd, Co, Cu, Ni, Pb, and Zn (analyzed by flame and GF-AAS) have been found to have increased since the late 1940s relative to population growth. In addition, levels of Cu, Pb, and Ni have been found to be potentially toxic [30].
Other studies have included analyses of living organisms and sediments, such as the study by Vázquez-Sauceda et al [31] conducted at San Andres Lagoon in the Gulf of Mexico. In this research, Cd, Pb, and Zn were analyzed in sediments, water, and oysters (via AAS) at five sites. Statistical analysis showed a positive relation between Cd and Pb levels in oysters and sediments; Cd and Pb pollution were also detected in water (measured by spectrophotometry) and sediments (measured by AAS) in the estuarine ecosystem of the Tigre River-San Andres Lagoon, with Cd levels reaching concentrations above the limits of adverse biological effects [32].
At the Alvarado Lagoon system, in Veracruz, concentrations and bio-accumulation of Hg have been determined in riverbank communities [33]. That study included the determination of Hg in sediment, water, fish, and people’s hair in the Papaloapan River basin. Particulate matter in the water was determined to be the main transport mechanism in the lagoon, while an association of Hg with organic matter in the sediments was found. Mercury was determined via gold amalgamation and quantification with a cold vapor atomic fluorescence spectrophotometer (CVAFS). The results showed that people who frequently consume fish and shellfish may be at risk
Pascual-Barrera et al [34] evaluated possible sources and the spatial distribution of heavy metals in sediments from three lakes within the petroleum extraction and processing zone of San Miguel, Chiapas. Samples were collected during the rainy and dry seasons. Spatial modeling of the concentrations (measured by ICP-AES) showed that discharge from a local petrochemical facility was the main source of heavy metals within the sediments.
Arcega-Cabrera et al [35] evaluated seasonal variations and the natural and environmental factors that influence the concentrations of heavy metals in the Chelem Lagoon, Yucatán; metals were measured by AAS in samples collected during the dry, rainy, and post-rainy seasons. Cd, Cu, Cr, and Pb concentrations presented statistically significant spatial and temporal variations, and concentrations of Pb, As, Cd, and Zn were found to be above sediment quality standards. Factor analysis (including organic matter, calcium carbonate, and fine fraction contents, As and metals concentrations) was applied to evaluate geochemical processes and PTE sources. In addition to direct wastewater discharge, groundwater was also found to be transporting metals to the lagoon. García-Ríos et al [36] determined the concentration and speciation (by sequential extractions) of Zn, Pb, Ni, Cu, Cd, Fe, and V (analyzed by ICP-AES) in sediments collected at another location (the Bahia de Chetumal) on the Yucatán Peninsula.
Hansen et al [37 proposed a methodology to estimate the mobilization of heavy metals (Cd, Cu, Cr, Fe, Mn, Ni, Ag, and Zn) from sediments to water; experiments were conducted by exposing a sediment sample to different redox conditions. The primary physico-chemical characteristics of the soil were determined using standard methods; concentrations of metals were analyzed with spectrophotometry. The adsorption of metals on pyrolusite and ferrihydrite was modeled using the PHREEQC v.2 geochemical program to evaluate the metals’ distribution between the solid and aqueous phases. The conclusion of the study was that in oxidized conditions, metals adsorb on the solid iron oxides of the sediment, while at negative Eh values, the oxides dissolve and release metals. At more reduced conditions and in the presence of sulfide, however, the concentration levels of dissolved metals were found to have decreased.
Fluvial sediments
In one study [38], sediments collected in a section of the Lerma River (one of the largest in Mexico) were collected and analyzed by flame and GF-AAS and XRF to evaluate the concentration and seasonal distribution of Cr, Ni, Cu, Zn, Fe, Pb, and As. The differences were evaluated between the dry and rainy seasons and among sampling stations by statistical methods; contamination degree was assessed by calculating EF (using Fe as the conservative tracer) and geoaccumulation index. The results, when compared with the protection criteria for freshwater sediments, indicated that all the analyzed elements except As and Cr had exceeded the LEL, while Cu surpassed the SEL at some of the sites.
In northwestern Mexico, Orozco-Durán et al [39] evaluated the distribution of Se, Mo, and U in sediment cores of around 1.5 m in length collected at the Colorado River delta, close to the Mexican-US frontier. ICP-MS was used to analyze the elements concentrations, organic carbon was analyzed with an infrared (IR) LECO elemental analyzer, and grain size was examined with a laser grain size analyzer. The concentration of Se in a few samples surpassed the probable toxic effect level. Organic carbon was used to assess the approximate age of the sediments. Wakida et al [40] also evaluated the pollution of heavy metals found in fluvial sediments at sites near the Mexico-US border. Statistical correlations, geoaccumulation index values, and the measured concentrations of Cr, Cd, Pb, and Ni along the Tecate River indicated an anthropogenic source, likely related to the local electroplating industry.
Gómez-Alvarez et al [41] conducted a study at the At San Pedro River, Sonora. The spatial distribution and chemical forms (obtained through sequential extraction) of Cd, Cu, Fe, Mn, Pb and Zn (analyzed by AAS) indicated an anthropogenic source of contaminants likely from a tailing pond as well as wastewater from Cananea City. The residual and Fe and Mn oxides fractions were identified as the most important storage of the metals. In addition, the concentrations of heavy metals in the non-residual fractions were found to be above sediment quality criteria.
Geochemical processes and the sources of heavy metals were determined in a study conducted by Jonathan et al [42] on sediments from the Pánuco River. A high level of erosion from the Sierra Madre Oriental was found to have increased the concentrations of Fe, Cr, Ni, Co, V, and Sr in one of the regions of the river basin. Trace elements were analyzed by ICP-OES and GF-AAS; texture, carbonates, and organic carbon in the sediments were also determined. Eh and pH were found to have influenced the concentration pattern of Fe and Mn; in addition, the presence of the local ferromanganese industry was found to have contributed to the acid- leachable fraction of these elements. Evidence of other anthropogenic sources of the analyzed metals was found, including pesticides, fertilizers, and refineries. Amezcua-Allieri et al [43] had previously evaluated nickel and vanadium concentrations in sediments from the same river and related these concentrations with the sediments’ toxicity. Dredging was found to have favored metal desorption to the water, thus increasing the metals’ bioavailability.
Many studies have been developed to assess the contamination degree and geochemical processes of PTEs in river sediments near mining-waste deposits. Arcega-Cabrera et al [44] determined the geochemical fractionation of Pb, as well as the 210Pb/214Pb ratios in sediments of the Cacalotenango and Taxco Rivers in the mining zone of Taxco, Guerrero. Statistical factor analysis of fractionated and total Pb, as well as geochemical fractionation, indicated a greater influence of anthropogenic sources on Pb concentrations, compared to natural sources (mineralization present in the area). The two main processes that control the yearly total Pb variation in both rivers are: (1) erosion with discharge, and (2) proportional dilution. Armienta et al [45] studied the geochemical behavior of Fe, Pb, Zn, Cu and As, close to a tailings deposit in a shallow branch of the Taxco River near Taxco City. Mineralogical (by XRD) and chemical determinations (by flame and hydride generation AAS) in sediments and tailings showed that sulfides oxidation and rainwater erosion had released metals and As into the sediments. Geochemical fractionation using the method proposed by Tessier et al [3] showed that the mobility decreased as Zn > Pb > Fe. Geoaccumulation index values indicated that sediments were strongly polluted with Zn, strongly to very strongly polluted with Pb, and moderately to strongly polluted with Fe. Espinosa and Armienta [46] noted the importance of the presence of limestone on the fractionation and environmental mobility of Zn and Pb in the same area. In another study [47], geochemical fractionation of heavy metals (Cd, Pb, and Zn) and As was also determined in the sediments of the Tolimán River at the historical mining zone of Zimapán in Hidalgo. Chemical analyses of metals and As were performed by GF-AAS; organic carbon, pH, total sulfur (by IR) and mineralogy (by XRD) were also determined in the samples. The spatial distribution of the concentrations showed evidence of the impact of tailings on the sediments’ contamination levels. Geochemical fractionation showed that pH decrease enhances the mobility of Cd, Pb and Zn due to the proportion associated with carbonates.
The San Antonio mining district in Baja California Sur is another zone that has been the subject of several studies. One study reported by Shumilin et al [48] examined the mobility of As in the dry stream sediments of the area. Samples were treated with various extractants to evaluate As mobility from the sediments. An examination of mineralogy (determined by XRD and IR) allowed for distinguishing differences in As-bearing minerals along the San Antonio arroyo. Reactive As was found to increase downflow while other metals showed distinct behaviors. Analyses were conducted via GF-AAS for As and Cd, while Cu, Fe, Mn, Pb, and Zn were determined via flame AAS.
Gutiérrez-Caminero et al [49] applied lead isotopes to identify toxic element sources in this same area. Isotopic determinations were performed on sulfides and scoria from abandoned mines, fluvial sediments, and igneous rocks. The isotope ratios of Pb showed evidence that most of the toxic element input into the sediments was related to massive vein- type mineralization, while fault-bounded mineralization was also an important contributor. In addition, average Mexican industrial lead was also found to contribute to the pollution in the area.
Another mining zone where environmental geochemistry studies have conducted is Santa Rosalía in the central Baja California Peninsula, where copper-mineral deposits and solid wastes from ore smelting have been identified as the primary sources of rare earth elements (analyzed by INAA) in marine sediments [50]. Contamination by mercury (determined by cold vapor AAS) has also been detected around the biggest copper mines at El Boléo near Santa Rosalía. Dispersal mechanisms include runoff from weathered materials and sulfides oxidation followed by chloride and organic complex formation [51]. Willerer et al [52] have also determined mercury concentrations (analyzed by cold vapor AAS) in sediments collected from the Marabasco River, its estuary, and Laguna de Navidad, Colima, where Hg-ore deposits and mines constitute potential sources of this element.
Aguilar-Hinojosa et al [53] studied the mobility and bioavailability of heavy metals in sediments collected from the Jaralito and Mexicana mining impacted streams in Sonora; total and sequential extraction concentrations were analyzed by AAS. Geoaccumulation index results showed moderate to strong contamination of Ni, Pb and Cu in sediments at Jaralito; among the Mexicana sediments strong contamination was shown by Cd, Cu, and Pb, and moderate contamination was shown by Ni, Pb, and Zn. Comparisons with LEL and SEL indicated that the areas represented an important environmental risk.
The mining area of Villa de la Paz-Matehuala, San Luis Potosí, has also been the subjected of numerous studies. Razo et al [54] reported on As, Cu, Pb, and Zn content levels in soils and dry sediments reaching several thousand mg/kg in the area. The concentrations of As and metals in water-storage ponds and sediments were found to have a general decrease from the pollution sources, such as tailings, waste-rock dumps, and slag piles. Gamiño-Gutiérrez et al [55] evaluated As and Pb contamination of urban soils in the same area. Bioaccesibility tests and total concentration results showed both natural and anthropogenic sources. The spatial distribution showed evidence that mine wastes were the main pollution sources. The determination of Pb in blood, As in urine, and micronucleated exfoliated cells assay in children showed that at least 20%-30% of the surveyed children had exposure values above maximum intervention concentrations and showed evidence of a genotoxic damage associated with As.
Castro-Larragoitia et al [56] investigated heavy metal and As sources, fates, and dispersion pathways at the Concepción del Oro mining zone in Zacatecas. Concentrations of heavy metals and As were determined in mining residues (waste rocks and tailings), fluvial sediments, stream waters, soils, and groundwater by ICP-MS; in addition, the main ions in groundwater were determined by Mexican Standard Methods including volumetry, turbidimetry, and flame AAS. Solid samples were characterized by XRD and energy- dispersive XRF. Tailings and stream sediments were treated with pH = 7 buffered bi-distilled water to estimate the mobilization potential of PTEs with rainwater. To evaluate the PTEs’ mobilization potential from polluted soils, samples were extracted with the solution used in the first step of the BCR sequential procedure; in addition, agricultural soils were treated with acetic acid to estimate the influence of biological activity in the PTEs’ transfer to plants. By the application of factor analysis and geostatistical methods, mining residues were identified as the primary contaminant sources. Heavy rains were found to play a major role in PTE dispersion.
Ramos-Arroyo et al [57] studied the processes that influence the natural decrease of As in a stream near abandoned silver mines in Guanajuato in order to evaluate the mobilization potential of PTEs from polluted soils. Geochemical interpretation of the results indicated that pH and redox conditions favored the oxidation of As(III) released from sulfide minerals. Multivariate statistical analyses indicated that the decrease of dissolved As was due to the association of As(V) with a solid phase that containing Fe, Mn, and Ca. Arsenic species, including As(III), DMAs(V), MMAS(V), and AS(V), were determined by liquid chromatography followed by ICP-MS.
Arcega-Cabrera et al [58,59] performed experiments to determine the kinetics of Pb and tetraethyl lead (TEL) release from fluvial sediments collected at the Taxco mining zone, under various pH conditions. Total Pb was determined by flame and GF-AAS, and TEL was determined by UV-visible spectrophotometry. The results of total Pb concentrations released over time from samples collected at two sites showed a linear behavior at pH = 8, exponential or linear behavior at pH = 7, and polynomial or exponential behavior at pH = 6, with higher release with decreasing pH. Fitted curves of TEL release with time from sediments collected at three sites showed that desorption shifted from linear to polynomial. Factor analysis revealed that wet deposition, runoff and leaching from the hydrological basin were the most probable sources of TEL in the Cacalotenango and Taxco Rivers.
Using experiments at different pH values, Martínez-Villegas et al [60] determined the influence of pH on Pb sorption on soil collected in Zacatecas. Lead concentrations in the solution were measured by differential pulse polarography and speciation was estimated with the MINEQL geochemical modeling program. The results showed increasing sorption with pH as well as behavior that followed the Langmuir and Freundlich isotherm models.
Soils
Concentrations, spatial distribution, pollution sources, and the geochemical behavior of PTEs in soils have also been studied in several areas of Mexico. In San Luis Potosí City, Carrizales et al [61] assessed the influence of a smelter on soil contamination and child exposure. Roughly 90% of the soil samples were found to contain Pb and As (measured by flame or GF-AAS, and Flow Injection for Atomic Spectroscopy-Hydride Generation-AAS [FIAS-HG-AAS] respectively) above EPA guidelines. Bioaccesibility determinations (in vitro gastric and intestinal tests) in 10 soil samples showed a potential exposure risk. Arsenic levels in the urine, and Pb levels in the blood of children living in the area were also analyzed; the results showed Pb concentrations above the action level in 90% of the children that originates mainly from exposure to soil and dust. Arsenic exposure doses calculated using Monte Carlo analysis were found to be above the EPA reference dose.
Tailings have also contaminated soils with heavy metals (Cd, Cu, Pb, Zn, and Fe) and As at a mining zone in San Luis Potosí. One study [62] related concentrations (measured by XRF and inductively coupled plasma optical emission spectrometry [IPC-OES]), and mineralogy (determined by XRD and Scanning Electron Microscopy with Energy Dispersive Spectroscopy [SEM-EDS]) with magnetic susceptibility, pH, and electrical conductivity. The results showed a positive correlation between hazard index (HI) and magnetic susceptibility and a negative correlation between pH and HI [62]. Similarly, another study [63] measured high concentrations of heavy metals (by flame AAS) in soils and sediments collected near mining sites in Aguascalientes. Sequential extraction and soil treatment in humidity cells were applied to estimate heavy metals’ environmental mobility. The results indicated that because of the physico-chemical characteristics of the substrates particularly neutral to alkaline pH, high organic matter content, and moderate to high cation-exchange capacity Cd, Cu, Pb, and Zn were not water leachable at these sites. Sequential extraction, however, showed that Cd and Pb may be mobilized because of these elements’ presence in the exchangeable and carbonate fractions [63].
Gutiérrez-Ruiz et al [64] examined the geochemical processes involved in the natural attenuation of As in soils contaminated by a lead smelter in Monterrey, Nuevo León. Total As and other trace elements were analyzed by ICP-AES, and As(V) was analyzed by ion chromatography with electrical conductivity detection. The results showed a much lower solubility of As in soils than in wastes. Geochemical modeling was applied to calculate saturation index values, and the results indicated that As solubility was controlled by the formation of solid Pb and Cu arsenate. This formation was also supported by the results of sequential extraction. Mineralogical characterization of the fine soil fraction by XRD, SEM and transmission electron microscopy coupled with energy dispersive X-ray spectroscopy showed evidence of the formation of metal arsenates. The formation of low-solubility Pb arsenate minerals (duftite and hydroxymimetite) was found to be the dominant process for the natural attenuation of As [64].
Another study [65] evaluated the influence of mining wastes on As concentration in soils in the Zimapán Valley. In addition, some samples were treated to determine the geochemical fractionation of As. A positive correlation of As concentrations with Cu, Pb, and Zn suggested the association of As with ore minerals. A very low proportion of As was in the most mobile water-soluble and exchangeable fractions. Chemical analyses were done by INAA, ICP-OES, and GF-AAS [65]. Duarte-Zaragoza et al [66] evaluated the contamination and spatial distribution of heavy metals (Cd, Cu, Mn, Ni, Pb, and Zn) in superficial soils near tailing heaps with various weathering degrees at the same mining zone. Concentrations were determined by AAS, and GIS software was used to estimate the contaminated area. Further studies in the same area [67] have focused on evaluating As and heavy metals mobility in soils near tailings by determining total and sequential extraction concentrations (by GF-AAS and ICP-AES) at the surface and at 40 cm depth during the dry and rainy seasons. Differences between As and heavy metals fractionation were found in soils that were influenced by each of three tailing heaps. Concentrations above the screening values established in Canada and the Netherlands showed that these soils represent a health threat to the inhabitants and the environment.
At Cerro de Mercado, Durango, Morales et al [68] evaluated the total and bioaccesible concentrations of As and Pb in soils affected by the exploitation of the Fe-oxide-rich ore deposit that produced tailing impoundments and waste rock dumps. As-bearing phases were identified using XRD, and ATR-FTIR, and metal analyses were performed with flame and HG-AAS. Total concentrations of As were found to exceed EPA and Agency for Toxic Substances and Disease Registry (ATSDR) trigger level for residential soil remediation. Bioaccesible fractions were found to vary from 0.7% to 18% of total concentrations. The presence of As sorbed on iron-oxide-rich materials was demonstrated by ATR-FTIR analysis. Bioaccesible fractions of Pb (2.1%-16% of total concentrations) were found to be below the reference concentrations established in Mexico. Groundwater pollution by As is a major problem in this area; in addition, fluoride is also present in high concentrations in wells in Durango [69].
Surface soils at La Comarca Lagunera (Durango and Coahuila states) northern Mexico have been sampled and analyzed to determine total, bioaccesible, and mobile concentrations of As and F to assess the elements’ environmental behavior [70]. Mineralogical determinations (via XRD and SEM-EDS) allowed for the identification of various As-bearing compounds such as mimetite-vanadite-like and lead-arsenate-like compounds, while fluoride was only found to be present in fluorite. Other parameters such as z-potential, pH, conductivity, cation exchange capacity, total organic content, textural composition and hydraulic conductivity were also determined in the soil. To assess As and fluorine bioaccesibility in the gastric and intestinal phases the Solubility/Bioavailability Research Consortium (SBRC) in vitro method was applied. Arsenic was determined by HG-AAS, and fluoride by UV-visible spectrometry. At many sites, total concentrations were found to exceed the background level for fluoride and the trigger criterion level for soil remediation for arsenic. While As was found to present low soil mobility, a high level of mobility was determined for fluorine. The bioaccessible percentages were found to reach 63% in the gastric phase for As and 46% for fluorine [70].
In another study, the spatial distributions of metals (Ni, Cr, Pb, Cu, Fe, Cd, V, Hg, Co, Se, and Mn) in soils and soil-derived dust was evaluated in towns and cities within the Yaqui and Mayo agricultural area of Sonora [71]. Concentrations were determined with a portable XRF, and by ICP-AES and ICP-MS. To assess the contamination degree, the geoaccumulation index and the integrated pollution index (IPI) were calculated. IPI is the mean of the pollution index (PI) of an element, where PIi = Ci/Bi, and Ci corresponds to the sample concentration and Bi to the background concentration. The results showed that Co, Mn, V, and Ni content related to power plants may pose a health risk to the population, mainly children, through ingestion and inhalation. The conclusion of the study was that risk assessments that consider concentrations in bulk soil may overestimate the risk when compared to the fine fraction.
In another study [72], the relevance of efflorescent salts formed in tailings were identified as an important source of heavy metals in nearby soils at the Nacozari mining zone, Sonora [72]. The study in this area included the analysis of the concentrations of metals (measured by a portable XRF) in mine tailings, efflorescence salts, soils and dust. Mineralogy was also determined by XRD. Multivariate PCA and clustering were used for data analysis. Enrichment factors showed highest average enrichment levels of Hg, followed by Cu and As, in residential soils. Geoaccumulation index values also reflected the same trend, with an Igeo of 6 corresponding to extreme contamination for Hg, and class 4 (heavy contamination) for As and Cu.
In one study [73], contamination by heavy metals (Cr, V, Ni, Hg, and Cd) in soils near a thermal power plant at Playas de Rosarito, Baja California was examined. Analyses of metals were performed by GF-AAS; organic matter, pH and texture were also determined in samples from topsoils and depth profiles. The study’s interpretations, including calculations of the Igeo, EF, and statistical analyses indicated that the use of fuel oil at the local thermoelectric plant had contributed to the metals’ accumulation in the soils [73].
In one study conducted in Mexico City [74], the spatial distribution of heavy metal concentrations (measured by XRF) in topsoils was defined with a GIS. The northern and central parts of the metropolitan area presented the highest contamination indices. The concentrations’ distribution and statistical analysis indicated that high concentrations of Pb, Cu, Zn, and Ba were related to vehicular traffic, while Co, Cr, Ni, and V originated from the parental rock. Only Cu and Zn had increased since 2003 [74].
Guédron et al [75] studied the contamination degree and geochemical behavior of metals in the Mezquital Valley, where raw wastewater has been applied to irrigate agricultural soil for decades. Mercury species dissolved in water were analyzed by cold vapor atomic fluorescence spectrometry. Major and trace metals, Se, and As were determined by ICP-AOS, and total particulate Hg was determined by AAS (AMA 254) following catalytic decomposition and gold amalgamation. Monomethylmercury was determined in solids after specific extraction, derivatization, and purge and trap-gas chromatograph AAS. The results were compared using various statistical tests. Wastewater canals were found to be transporting high concentrations of methylmercury, lead, and other metals, while downstream, in the Tula River, concentrations showed a notable decrease. Metals were found to have been retained in the draining tilled layer due to the oxic conditions and slightly acidic pH, which favor metal adsorption and co-precipitation and, possibly, mercury demethylation.
Maldonado et al [76] investigated the influence of wastewater irrigation patterns on heavy metal content (determined by ICP-OES) in soils near Chihuahua City. The concentrations of Na, K, Cd, Pb, Ni, Cr, Cu and Fe, besides pH, electrical conductivity and organic matter in samples collected at three depths with variations of wastewater exposure were determined. Statistical methods showed soil type as the only variable to have a significant difference among the sampled sites. The results showed evidence of anthropogenic and natural sources of the metals.
In another study [77], concentrations of the radioactive elements 222Rn, 137Cs, 235U, 226Ra and 40K, measured with solid-state nuclear track detection (SSNTD using nitrate cellulose foils) and a High Purity Germanium (HPGe) gamma detector, were determined in forest soils near the Nuclear Research Center in central Mexico. The results showed moderate 235U and 226Ra punctual contamination.
Groundwater
The evaluation of groundwater contamination degree, sources of contaminants, and hydrogeochemical processes has been accomplished in many areas of Mexico. This is particularly relevant because most of the country’s drinking water is abstracted from aquifers. Effective actions are thus required in zones where PTE concentrations are above drinking water standards to protect the potentially exposed populations. Many studies have included chemical and isotopic analyses and interpretations that have considered the hydrogeological framework; other studies have focused on the influence of aquifer systems’ hydrogeological characteristics on water quality and contaminant presence. This section of the paper will present the main objectives, methods, and results of selected cases as a way of providing an overview of the contamination issues, with a focus on those that are more closely related to environmental geochemistry.
Fluoride and arsenic concentration levels and sources in groundwater have been an important research topic due to the presence of these toxic elements in aquifers in concentrations above drinking water standards in many areas of the country. Environmentally hazardous levels of arsenic, fluoride, and heavy metals in groundwater due to natural processes as a result of water-rock interactions are present worldwide. In many cases, these levels also result from human action; in other cases, both natural and anthropogenic sources coexist in the same zone. The identification of the specific sources is important in order to take effective actions to protect the environment, as well as the people that may be directly or indirectly exposed to hazardous levels of these contaminants. At various sites, As and F occur at concentrations above drinking water standards and guidelines in the same water source. Review papers have provided an overview of the problem, including health effects, concentrations, and sources of As and fluoride [69,78,79,80]. Birkle et al [81] and López et al [82] have reported on the occurrence and geochemical processes influencing As contamination in various geothermal zones of the country. Several examples of studies conducted at specific zones that exhibit As and/or fluoride contamination are presented below.
La Comarca Lagunera in Durango and Coahuila states has been one of the most studied areas, focusing on health problems due to ingestion of As polluted water that were identified in 1958 [83], and on the sources and processes producing the As contamination of groundwater. The origin of As in the sedimentary aquifer, however, is still a matter of debate. Arsenic pesticide use has been suggested as the origin of As pollution [84]. Molina [85] proposed several geochemical processes in different zones of the aquifer, such as desorption of arsenic retained on clays, the release of As from the dissolution of iron and manganese oxides, and the oxidation of sulfides. Ortega-Guerrero [86] after interpreting chemical and isotopic determinations and groundwater flow modeling, postulated evaporation as the main process producing high As concentrations in the southeastern-most part of this area. Mejía-González et al [87] have proposed the release of As from sediments in the water due to increase in pH. Ortega-Guerrero [88] evaluated the aquitards’ geochemical influence on As enrichment at the edges of the Comarca Lagunera. Geochemical modeling (PHREEQE) was used to determine saturation indices for various minerals in the granular and carbonate aquifers and in the aquitard. The results indicated that the advance of As-rich groundwater to the main granular aquifer was due to a reversal of hydraulic gradients as a result of intensive groundwater exploitation, and to the decrease of freshwater runoff from dam construction in the main rivers of the area. In these studies, arsenic and metals were analyzed by HG-AAS, ICP-MS, or ICP-AES.
Concentrations of As and F above Mexican drinking water standards (0.025 mg/L and 1.5 mg/L, respectively) have also been measured at the alluvial aquifer system in Chihuahua [89,90]. Espino-Valdés [89] analyzed arsenic by ICP-AES in groundwater samples collected from the Meoqui-Delicias aquifer and determined other hydrogeochemical parameters. An interpretation of the concentrations distribution within the hydrogeological and geological framework indicated a natural geogenic source related to the recharge flow from mountains presenting arsenopyrite deposits, as well as from the contact of water with the aquifer sediments. In addition, at Julimes municipality, geothermal water and high evaporation rates were also found to be responsible for As contamination. Mar Camacho et al’s review [80] which included information from water, soils, and sediments, reported natural (related to volcanic processes) and anthropogenic (related to mining and smelting) sources in the Mexican states of Chihuahua and Coahuila as well as the bordering states of the southwestern United States. The review also included the influence of oxidation and pH on the mobilization of As in those areas, as well as health effects and water treatment procedures.
Reyes-Gómez et al [90] studied the co-occurrence of fluoride and As in the central part of Chihuahua. Arsenic content in rocks, sediments and groundwater was determined by AAS and ICP-AES, and fluoride was quantified by potentiometric analysis with ion selective electrodes. Petrographic analyses showed the presence of fluoride in fluorapatite. Distribution maps depicted temporal (from 2003 to 2010) and spatial concentration distributions of As and F; concentrations of As and F showed a similar trend. The measured values of pH and Eh indicated that As predominated as HAsO4 2- in groundwater. The authors proposed a geochemical conceptual model based on the chemical characteristics of rocks, sediments, and water to reflect the mobility of As and F in groundwater. Highly fractured volcanic rocks and alluvial fans at the base of the mountains were identified as possible aquifer recharge zones. The alluvial fans were found to contain rhyolites and shales with As and fluorapatite. Weathering releases these elements from the lithology of the area.
Arsenic contamination is related to natural and/or anthropogenic sources in mining zones. At Zimapán, Hidalgo, two anthropogenic sources and one natural source have been identified as the origin of As groundwater pollution in the aquifer system. Rocks, mining wastes, soils, and sediments have been analyzed by XRF, ICP-OES, and AAS; mineralogy has been determined by XRD and SEM-EDS; chemical analyses of water have included the main ions determined by standard methods; As and heavy metals by HG-AAS and ICP-OES, as well as isotopic analyses (of δ18O and δ2H in water, and δ34S in dissolved sulfates) by MS. The interpretation of the results within the hydrogeological framework has allowed for the definition of the contamination degree as well as for the arsenic source and mobility. Water interaction with As-bearing minerals in the aquifer matrix releases As to the deep fractured limestone aquifer, while acid mine drainage from tailings and the infiltration of As-enriched water from smelter stacks has contaminated the shallow aquifer [91,92].
Abandoned mines may also affect groundwater quality. At Huautla, Morelos, the mines (which closed in the early 1990s) were flooded, and the water was then used by local people and livestock. Esteller et al [93] found high concentrations of As, Fe, Mn, Pb, Cd, and fluoride in groundwater samples in this area. In their study, arsenic levels were determined by HG-AAS and heavy metal concentrations were determined by GF-AAS. The main ions were analyzed by standard methods, including volumetry, spectrophotometry, and flame AAS. The interpretation of their results included identification of water type, basic statistics, and geochemical modeling using PHREEQC and AQUACHEM to determine chemical speciation, saturation indices, and water-rock interaction processes. In addition, mineralogical determinations (by XRD) were included in the interpretations. Various geochemical processes were proposed as the origin of As pollution, including sulfides oxidation and the reductive dissolution of iron oxides and hydroxides. After release, arsenic mobilization may be controlled by other processes such as adsorption on Fe hydroxides or calcite, with the latter showing a higher retention capacity.
Martínez-Villegas et al [94] studied As source and the geochemical behavior of a perched aquifer in the mining area of Santa María de la Paz, San Luis Potosí. Major cations in water were analyzed by AAS and anions by colorimetry; concentrations of As and other trace elements were determined by ICP-MS. Soil samples were collected and analyzed with a portable XRF; the six most polluted samples were then analyzed by ICP-OES. Soil mineralogy was determined by XRD. Arsenic in leachates from soil extraction tests was also determined. PHREEQC was used to compute saturation index values and to estimate aqueous speciation; the results were utilized to construct an activity ratio diagram. The geochemical interpretations also involved pH-pe diagrams and bivariate plots. Arsenic origin in groundwater was ascribed to the dissolution of calcium arsenates present in smelter residues, although diagenetic formation of calcium arsenates was found to prevent the further mobilization of As in the aquifer.
The mining area of San Antonio-El Triunfo near La Paz, Baja California Sur, has been the subject of several studies on sediments, groundwater and mining residues. Wurl et al [95] in 2014 reported on the presence of As and investigated possible As sources in the southern part of the district. In that study, physico-chemical determinations and arsenic, cyanide, and boron analyses of 29 groundwater samples were conducted in certified laboratories, where As was measured by ICP-MS. The results were interpreted by means of hydrogeochemical plots and relations, Eh vs pH diagrams, statistical correlations among trace elements, and cluster analysis. A lower impact of old mining activities was found in this area compared to the impact reported in the northern part of the region. In addition, the chemistry of samples from geothermal water indicated that the hydrothermal fluids had not significantly contributed to the presence of As in the area.
Birkle et al [81] evaluated the sources and geochemical processes of As in various Mexican geothermal zones by statistical and hydrogeochemical interpretations of published data as well as As analysis (by AAS) of specific water samples. Hierarchical cluster analysis (HCA) showed evidence that arsenic was linked to typical geothermal species such as lithium, silica, and boron. Secondary water-rock interaction processes were also found to have influence on As occurrence. A lack of arsenic solubility control was shown by speciation modeling using the PHREEQC and SOLVEQ programs. Rock leaching at high temperatures was found to release As into the geothermal water. Temperature was found to influence As release in magmatic-type reservoirs but not to have such an influence on sedimentary-type reservoirs.
Various negative health effects from the chronic ingestion of fluoride contaminated groundwater have been reported in San Luis Potosí State [69,78]. High fluoride concentrations (measured by ion selective electrode) have been found in wells in San Luis Potosí City; the fluoride was found to have originated from water-rock interactions in a regional deep flow through the fractured volcanic aquifer, which may also have polluted the non-thermal shallower aquifer. Management of the abstraction regime may help to control fluoride concentrations in the future by considering fluorite solubility [96]. The uranium and arsenic enrichment mechanisms of groundwater have also been determined near San Luis Potosí City in the Villa de Reyes volcano-sedimentary graben by Banning et al [97]. To that end, volcanic rock samples were characterized by sequential X-ray spectrometry and ICP-MS, and sediments were analyzed by INAA or ICP-OES. U and As were determined in solutions from a four-step sequential extraction of four sediment samples by AAS, and Ca and Mg were determined by ICP-OES. The highest As and U contents were found in rhyolites. The hydrogeochemical results, particularly As/U ratios and rare-earth element signatures, indicated that the dissolution of volcanic glass from high silica (>63 weight % SiO2) rocks is the dominant process releasing As and U into groundwater.
Concentrations above drinking water standards have been measured in the groundwater of the Guanajuato’s Cuenca de la Independencia basin. Mahlknecht et al [98] developed an study to determine the processes involved in the geochemical evolution and mineralization of the area by means of chemical and isotopic (δ 18O, 13C, and 3H) analyses of groundwater, mineralogical determinations of rocks from boreholes by XRD, geochemical mass-balance modeling (using PHREEQC), and multivariate statistical analysis. The interpretation of the results led to the conclusion that the weathering of rhyolites and the oxidation of As-bearing minerals produces the high As and F concentrations. Ortega-Guerrero [99] also investigated the concentrations, distribution and sources of As and fluoride in the same basin. Chemical (major and trace elements) and isotopic (δ18O, δ2H, 13C/14C, and 3H) determinations were interpreted in the hydrogeological framework in that study. Analyses were conducted in Canada using ICP-MS and ion chromatography. The hydrogeochemical and isotopic results indicated that As originates from the dissolution of silicates, while fluoride is related to the dissolution of fluorite and silicates, thermal water, and a longer residence time of the water.
Morales-Arredondo et al [100] studied the hydrogeological and geothermal factors related to the origin of arsenic and fluoride in another area of Guanajuato, at Juventino Rosas municipality. The chemical characteristics of the water were analyzed by Mexican and international standard methods, fluoride was analyzed by potentiometry with a selective electrode, and As was analyzed by HG-AAS; the isotopic composition of the water (δ18O and δ2H) via MS as well as radon concentration in soils (using alphaGUARD equipment) were also determined. The interpretation of the results within the geological and hydrogeological framework using hydrogeochemical plots and statistical methods allowed for a relation of the water type with the concentration ranges and circulation patterns of the groundwater. Rhyolite units appeared to be the most probable source of As and fluoride in the region.
In one study [101], inverse geochemical modeling was used to determine the geochemical groundwater geochemical processes at work and to evaluate the influence of anthropogenic and natural sources of salinity on the aquifer system of Guadalajara, Jalisco. The study included (1) a proposed conceptual hydrogeological model, (2) hydrogeochemical characterization, and (3) an analysis of the results by geostatistical tools, scatter plots, statistical treatment of data, silicate stability diagrams, calculation of saturation indices, and inverse modeling. PHREEQC was used for inverse modeling along three flow paths considering the mineralogy and lithology of the studied area. Water samples were sent to Canada for the analysis of cations (by ICP-MS) and anions (by IC). The results of the study allowed for the conclusion that rock weathering (primarily of silicates and evaporites), as well as anthropogenic activities account for the chemical characteristics of the aquifer system. Fluoride contamination was found to have resulted from fluorite dissolution and ion exchange on hydroxyl silicates. In addition, the precipitation of calcite was found to influence the dissolution of fluorite in hydrothermal waters. Nitrates resulted from the use of fertilizers or organic products, septic tanks and manure in the rural area, while wastewater and the leaching of waste disposal were found to be the contaminant sources in urban areas.
Gárfias et al [102] determined the sources and processes that increase solutes, particularly sulfate, in the Puebla aquifer system by geochemical and isotopic analyses interpreted within the hydrogeological context. The main anions in the water were analyzed by IC and cations were analyzed by ICP-AES in the laboratory, while alkalinity, pH, conductivity and dissolved oxygen were measured in the field. Isotopic determinations (by MS) included δ18O, δ2H in the water, and δ34S and δ18O in dissolved sulfates. Heavy pumping was found to have influenced the intrusion of mineralized water into the aquifer. The isotopic characteristics of sulfate indicated that the source of the sulfate in high sulfate waters is the dissolution of gypsum and anhydrite.
The origin of sulfate contamination in groundwater in Colima was examined by Horst et al [103]. Chemical determinations included major and minor cations and trace elements which were analyzed by ICP-MS and ICP-OES, and anions which were analyzed by IC. Saturation indices were calculated using PHREEQC. Isotopic analyses of sulfur and oxygen in dissolved sulfates and oxygen and hydrogen in water, jointly with geochemical indicators (specifically, high Sr concentrations), indicated that high sulfate concentrations had resulted from the dissolution of evaporites, although two samples seem to have been influenced by volcanic gases from Colima volcano [103].
In another study [104], isotopic determinations of chromium (δ53Cr) contributed to an understanding of the mobility and geochemistry of Cr in the Leon Valley aquifer which has been polluted by natural and anthropogenic sources (via pyroxenite weathering and industrial wastes respectively).
Final Remarks
Many environmental geochemistry studies have been conducted inland and on the coasts of Mexico; those reported in this article are shown in Fig. 1. The geographical distribution across nearly the entire country is evident from the figure, although some places have been the subject of several studies while other areas have not. Analytical chemistry has been applied in each of these studies but with variations in the frequency of the methods the researchers have applied for the quantification of PTEs (Fig. 2). Most of the studies (54%) measured the potentially toxic metals and metalloids using AAS (flame, hydride generation, graphite furnace, or cold vapor), followed by ICP-OES (28%), ICP-MS (14%) and INAA (3.5%). The percentages differ among substrates, however, with the highest difference being among AAS and other techniques for marine sediments and the lowest being for soils. Other analytical techniques have also been applied in environmental geochemistry studies, primarily potentiometry, XRF, XRD, SEM-EDS, IC, IR, UV-visible spectrophotometry, and volumetry, in addition to radioactive and isotopic methods. Few studies have reported the direct determination of the elements’ speciation. Interpretations of the geochemistry of these elements have also been done using methods that are commonly applied in analytical chemistry including Eh vs pH diagrams, saturation index calculations and the unraveling of geochemical processes in relation to specific physico-chemical conditions.
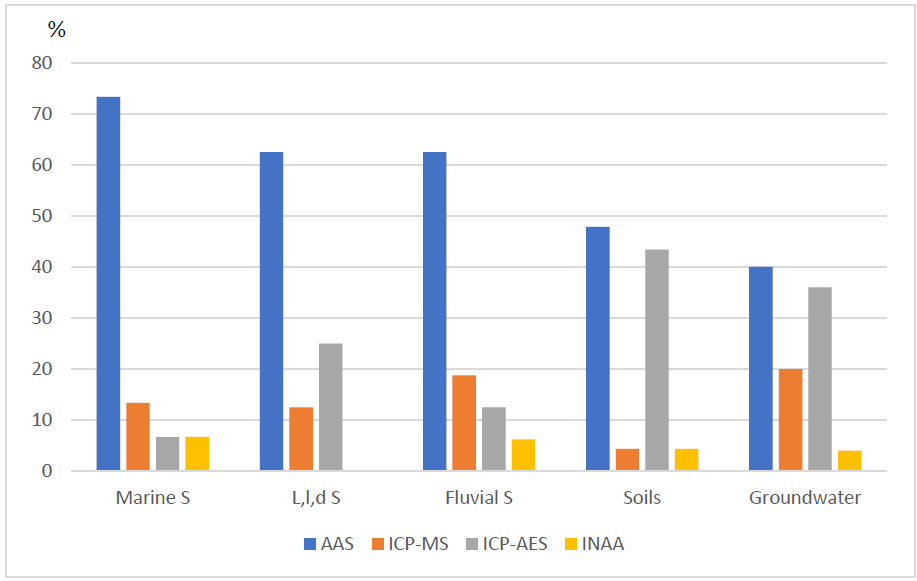
Fig. 2 Analytical methods used for the analysis of PTEs. Marine S = Marine sediments, L,l,d S =Lakes, lagoons and dams sediments, Fluvial S= Fluvial sediments.
Studies related to the contamination of solids usually include depictions of spatial concentration distributions, calculations of pollution indicators (such as enrichment factors and geoaccumulation indices), statistical treatment of physico-chemical determinations, evaluations of concentrations with respect to environmental guidelines or standards, and the identification of potential anthropogenic and natural sources. Many studies also include the treatment of samples with extraction procedures to assess the environmental mobility or bioavailability of PTEs. Isotopic determinations are also used to assess historical trends and to evaluate pollution sources at some sites.
Most of the groundwater studies have included the determination of principal physico-chemical parameters (such as main ions, pH, T, conductivity, and redox potential) in addition to analyzing PTEs in the water. Results have usually been interpreted within the hydrogeological and geological framework of the zones under consideration and by different relations among the concentrations of chemical species. In addition, some investigations have related the geochemical behavior and sources of the PTEs with their speciation determined from Eh-pH diagrams. Statistical tools have also been used in many studies. Isotopic data, primarily from stable isotopes of O, H, S, C, and Cr, has also been used to evaluate the geochemical processes and sources of contaminants.
Although the present review has shown that geochemical studies have been carried out by applying a variety of analytical techniques with successful results across many parts of Mexico, the pollution problems have not yet been solved in most of these areas. This is the case in the Lerma River (Central Mexico), Concepción del Oro (Zacatecas), the Chelem Lagoon (Yucatán), the San Antonio area (Baja California Sur), the Cerro de Mercado area (Durango), the Cuenca de la Independencia basin and Juventino Rosas municipality in Guanajuato, among others. Any anthropogenic sources that are identified must be eliminated or remediated, and alternatives must be provided in naturally or anthropogenic contaminated zones to avoid exposing the population to toxic elements. In addition, more laboratory facilities should be established to allow for the study of areas that have not yet been the subject of research and to continue to evaluate the contamination present at other sites. Doing so will require the involvement of more researchers and an increase in funding for the creation and maintenance of laboratories, and hiring of qualified laboratory personnel. While research on treatment alternatives and remediation options for pollution sources has also been conducted in the country, the authorities of Mexico as well as members of society in general should be aware of the relevance of these problems and become involved in taking the appropriate actions to benefit the environment and to protect the country’s public health.











 text new page (beta)
text new page (beta)