Introduction
3-Alkylindoles are important heterocyclic compounds and have been widely used for the production of a variety of chemicals [1-4]. Nowadays, numerous methods have been developed for the preparation of these molecules, and a classical method constitutes the alkylation of indole [5]. Conventionally, the common protocol for the assembly of these compounds is the alkylation of indoles with halides [6,7]. However, these protocols suffered from some drawbacks such as the use of stoichiometric amount of Lewis acid and generation of vast amounts of corrosive wastes. In order to overcome these problems, many efforts have been developed for the catalytic alkylation of indoles, and various compounds were used as nucleophiles including imines [8,9], nitroalkenes [10,11], ketones [12-15], aldehydes [16,17], epoxides [18-21], alcohols [22-25], N-Boc aminals [26], 2-enoylpyridine-N-oxides [27], p-quinols [28], and others [29-33]. Among these nucleophiles, epoxides and alcohols have attracted more interesting owing to the fact that their alkylation reactions with indoles are atom economic [18-25]. However, most of these methods still suffer from several shortcomings such as elevated reaction temperatures, long reaction times, high cost, and unsatisfactory yields. Therefore, there is an enormous demand for the development of environmentally benign and efficient methods for the direct catalytic alkylation of indoles with epoxides or alcohols that address these drawbacks under mild reaction conditions.
Ionic liquids (ILs) are well-known green reaction media and catalysts because of their outstanding and unique properties [34-36]. Examples of their applications in the alkylation of indoles were reported [15,37]. Although their good catalytic activities in the reaction, they have some problems such as partially ILs wasted and difficulties in separating catalysts from products after the reaction. Immobilized ILs have the attractive features of ILs and heterogeneous catalysis, such as easy catalyst recovery, high efficiency, high stability and recyclability. Thus, this problem can be overcome by immobilizing the traditional IL on solid supports [38-43]. Various methods have been developed for immobilization of ILs on solid supports such as alumina, titania, silica, etc, and silica was found to be one of the most effective one [42]. In the present study, a series of silica supported functionalized ionic liquids have been synthesized and characterized, which exhibited good chemical and thermal stability, and were applied as a type of novel heterogeneous catalyst in the preparation of 3-alkylindoles by alkylation of indoles with epoxides or alcohols (Scheme 1).
Results and discussion
The supported ILs catalysts, anion-NMPIL@SiO2, were prepared according to reported process [40-48] in three steps, Scheme 2 presents the synthetic strategy for the supported ILs. Cl-NMPIL was firstly prepared by the reaction of 1-methylpyrrolidin-2-one with (3-chloropropyl) triethoxysilane in toluene under refluxed conditions, and then the anion-exchange reactions were happened by treating Cl-NMPIL with LiNTf2, NaBF4, KPF6, ZnCl2, AlCl3, H2SO4, H3PMo12O40 or CF3SO3H to form anion-NMPIL, followed by the covalent attachment of ILs onto the surface of silica to afford the corresponding supported ILs anion-NMPIL@SiO2.
The characterization of the supported ILs were using FT-IR, XRD, UV-Vis, SEM, EDX and TG analysis. The FT-IR spectra of the supported ILs are shown in Fig. 1. The characteristic bonds at about 3015, 2820, 1775, and 1560 cm-1, which were assigned to the butyl inner amide ring [44]. The peaks observed at about 1080 and 970 cm−1 were belonged to the stretching vibration of Si-O-Si. The peaks at about 3500, 2970 and 1310 cm-1 are attributed to C-H stretching of alkyl chain. The peaks observed at 1358, 1192, 1050 cm-1 (Fig. 1a), 1718, 875 cm−1 (Fig. 1b), 827 cm−1 (Fig. 1d), 1542, 1450 cm−1 (Fig. 1e), 1065 cm−1 (Fig. 1f), 510 cm−1 (Fig. 1g), and 1025 cm−1 (Fig. 1h), related to vibrational modes of NTf2, HSO4, PF6, ZnCl3, BF4, AlCl4 and CF3SO3, respectively. Fig. 1c displayed characteristic peaks at 1068, 972, 876, and 798 cm−1 were related to the Keggin structure of PMo12O40 [49].

Fig. 1 FT-IR spectra of NTf2-NMPIL@SiO2 (a), HSO4-NMPIL@SiO2 (b), H2PMo12O40-NMPIL@SiO2 (c), PF6-NMPIL@SiO2 (d), ZnCl3-NMPIL@SiO2 (e), BF4-NMPIL@SiO2 (f), AlCl4-NMPIL@SiO2 (g) and CF3SO3-NMPIL@SiO2 (h).
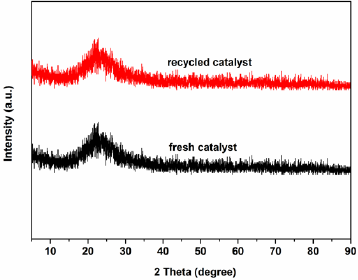
Fig. 2 displayed the XRD diffractograms of the supported ILs. All of the supported ILs had characteristic peak at 2θ ≈ 22.3°, which was the amorphous nature of the SiO2 support [41]. The absence of obvious characteristic peaks of anions of ILs in the XRD patterns suggests that the anion is well‐dispersed on the surface and in the channels of the SiO2 support. Fig. 3 showed the diffuse reflectance UV-Vis spectra of the supported ILs. All the supported ILs exhibit a peak around 235 nm, which are assigned to the O-Si transition [38]. In addition to this peak, another peak appeared at around 500 nm in Fig. 3c, which is likely to be associated with O-Mo ligand to metal charge transfer [50].
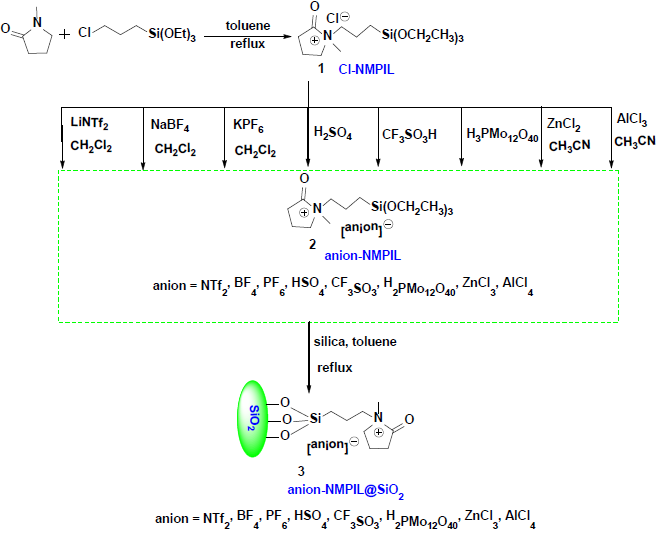
Fig. 2 XRD diffractograms of NTf2-NMPIL@SiO2 (a), HSO4-NMPIL@SiO2 (b), H2PMo12O40-NMPIL@SiO2 (c), PF6-NMPIL@SiO2 (d), ZnCl3-NMPIL@SiO2 (e), BF4-NMPIL@SiO2 (f), AlCl4-NMPIL@SiO2 (g), and CF3SO3-NMPIL@SiO2 (h).
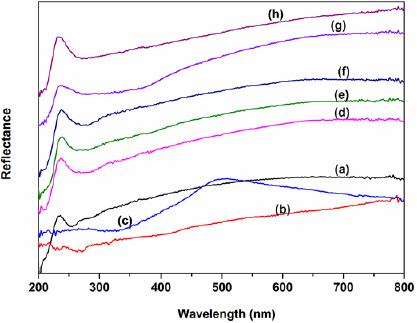
Fig. 3 UV-Vis spectra of NTf2-NMPIL@SiO2 (a), HSO4-NMPIL@SiO2 (b), H2PMo12O40-NMPIL@SiO2 (c), PF6-NMPIL@SiO2 (d), ZnCl3-NMPIL@SiO2 (e), BF4-NMPIL@SiO2 (f), AlCl4-NMPIL@SiO2 (g), and CF3SO3-NMPIL@SiO2 (h).
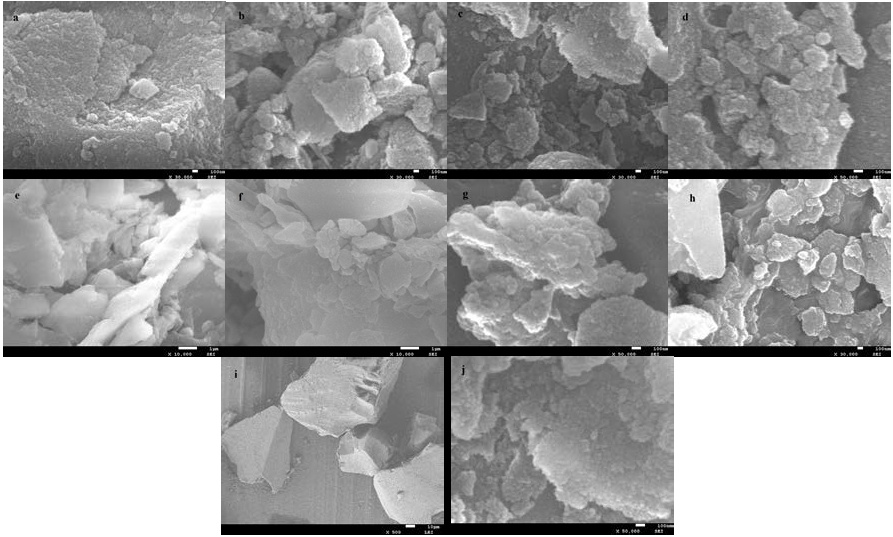
The surface morphology and chemical composition of the supported ILs were analyzed by SEM and EDX. SEM images of the supported ILs and silica gel are shown in Fig. 4a-i, it clearly observed that all the ILs particles were well-defined, and the particle size of the ILs is smaller to that of silica gel. Compared to silica gel, the surface of the supported ILs turned smooth and soft, and the images of the supported ILs shows highly uniform particles. Fig. 5 displayed the EDX analysis of the supported ILs. EDX obtained from SEM showed the presence of the expected elements in their structure.
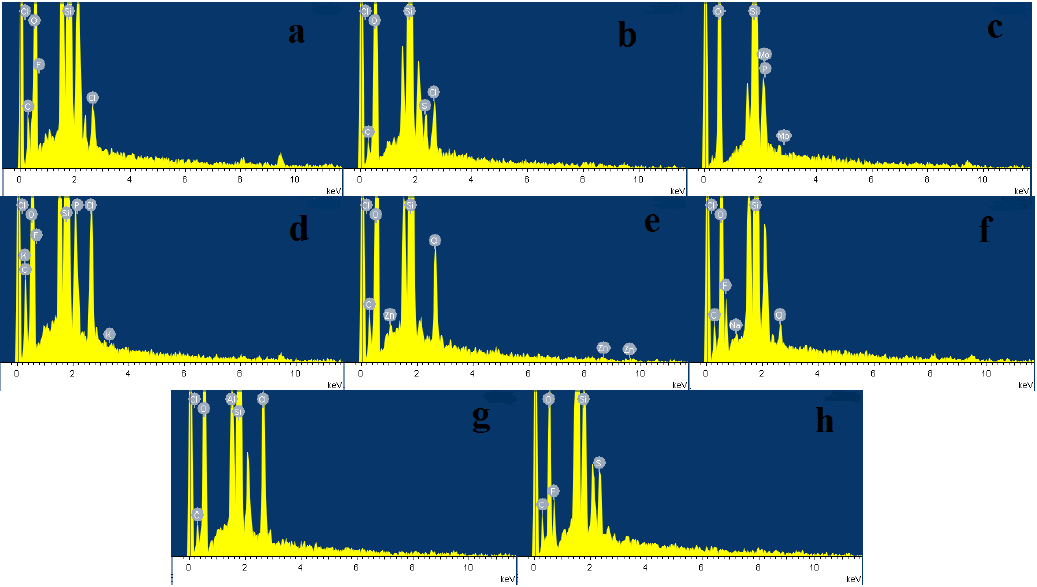
Fig. 5 EDX images of NTf2-NMPIL@SiO2 (a), HSO4-NMPIL@SiO2 (b), H2PMo12O40-NMPIL@SiO2 (c), PF6-NMPIL@SiO2 (d), ZnCl3-NMPIL@SiO2 (e), BF4-NMPIL@SiO2 (f), AlCl4-NMPIL@SiO2 (g), and CF3SO3-NMPIL@SiO2 (h).
The overall thermal stability of the supported ILs were conducted through TG analysis. In the TG curve, as shown in Fig. 6, the observed organic materials weight loss of the supported ILs showed a mass weight loss of ~17.5 % (Fig. 6a), ~52.8 % (Fig. 6b), ~11.2 % (Fig. 6c), ~14.3 % (Fig. 6d), ~20.4 % (Fig. 6e), ~13.1 % (Fig. 6f), ~29.8 % (Fig. 6g), ~39.4 % (Fig. 6h) on heating to 800 °C. The small weight loss before 210 °C is related to the desorption of adsorbed water, indicating a considerably thermal stability up to 210 °C. There existed drastic weight loss between 210 and 460 °C is attributed to the breakdown of the organic moieties. From the curves depicted, it demonstrated that the supported ILs are thermally stable below about 210 °C.

Fig. 6 TGA profiles of NTf2-NMPIL@SiO2 (a), HSO4-NMPIL@SiO2 (b), H2PMo12O40-NMPIL@SiO2 (c), PF6-NMPIL@SiO2 (d), ZnCl3-NMPIL@SiO2 (e), BF4-NMPIL@SiO2 (f), AlCl4-NMPIL@SiO2 (g), and CF3SO3-NMPIL@SiO2 (h).
In order to optimize the reaction conditions and test the catalytic activities of the synthesized supported ILs, initially we selected the alkylation of indole with 2-phenyloxirane for the preparation of 2-(1H-indol-3-yl)-2-phenylethanol as a model reaction and the proper chosen of ILs were examined for this reaction. A series of supported ILs catalysts such as NTf2-NMPIL@SiO2, HSO4-NMPIL@SiO2, H2PMo12O40-NMPIL@SiO2, PF6-NMPIL@SiO2, ZnCl3-NMPIL@SiO2, BF4-NMPIL@SiO2, AlCl4-NMPIL@SiO2, and CF3SO3-NMPIL@SiO2, were probed in the reaction (Table 1, entries 1-8). Among the supported ILs, H2PMo12O40-NMPIL@SiO2 demonstrated the best results in terms of product yield and selectivity (Table 1, entry 3). The contrast experiments were studied with bulk ILs catalysts, such as HSO4-NMPIL, H2PMo12O40-NMPIL, CF3SO3-NMPIL (Table 1, entries 9-11). Results show that the supported ILs showed much higher reaction efficiency than that of the bulk ILs in terms of product yield and selectivity, which might be due to the increase of the surface area of the catalyst after supported on SiO2 carrier. Therefore, H2PMo12O40-NMPIL@SiO2 was chosen as the suitable catalyst in the subsequent experiments.
Table 1 Preparation of 2-(1H-indol-3-yl)-2-phenylethanol by alkylation of indole with 2-phenyloxirane over various catalysts. a
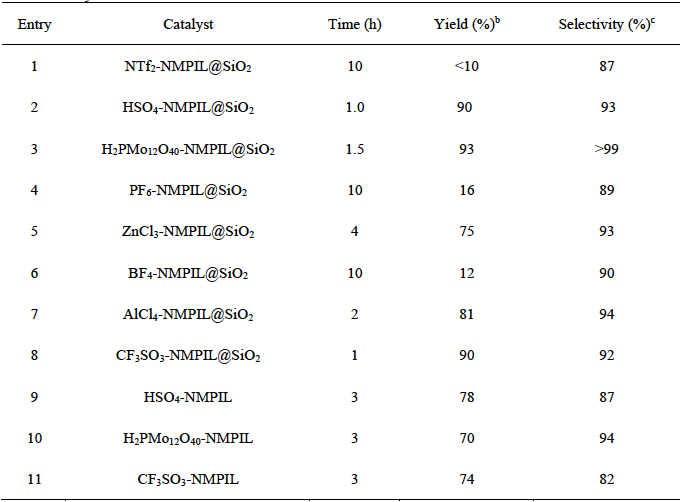
a Reaction conditions: indole (0.1 mol), 2-phenyloxirane (0.1 mol), CH2Cl2 (20 mL), catalyst (1.2 mol%), rt.
b Isolated yield.
c Isolated 2-(1H-indol-3-yl)-2-phenylethanol selectivity.
The influence of different solvents on the alkylation were investigated (Fig. 7). Solvents such as CH3CN, ether, hexane provided slightly lower yields, whereas other solvents such as ethyl acetate, water, THF, and acetone produces bad yields and selectivities. The studies revealed that the reaction medium has important influence on the reaction, and CH2Cl2 was identified as the optimal solvent, with the yield 93% and selectivity > 99% being achieved after 1.5 h.
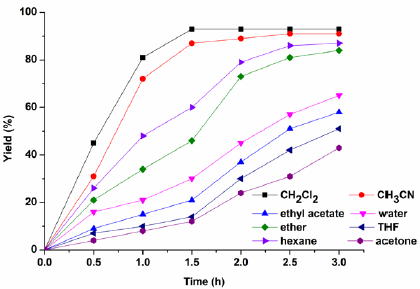
Fig. 7 Effect of solvent on the alkylation. Reaction conditions: indole (0.1 mol), 2-phenyloxirane (0.1 mol), solvent (20 mL), H2PMo12O40-NMPIL@SiO2 (1.2 mol%) at room temperature.
It was found that the alkylation was significantly affected by the amount of supported catalyst (Fig. 8). The reaction did not perform in the absence of H2PMo12O40-NMPIL@SiO2. As expected, the reaction activity was strengthened with the increase of the catalyst amount, the product yield and selectivity raised with the amount of catalyst increased to 1.2 mol%. However, there was no substantial change in the yield and selectivity with further increasing the amount of catalyst, while decreased the amount of catalyst resulted in lowering the yield and selectivity. Therefore, the best result was obtained by carrying out the reaction with a catalyst amount of 1.2 mol%.
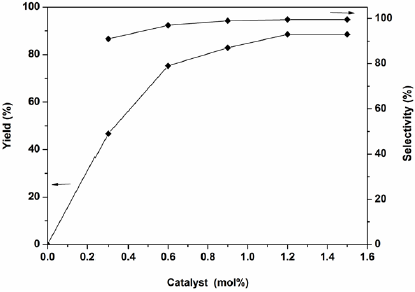
Fig. 8 Effect of the amount of catalyst on the alkylation. Reaction conditions: indole (0.1 mol), 2-phenyloxirane (0.1 mol), CH2Cl2 (20 mL), in different amounts of H2PMo12O40-NMPIL@SiO2 at room temperature for 1.5 h.
The heterogeneous catalyst H2PMo12O40-NMPIL@SiO2 could be easily separated and recovered by filtration after the reaction. The alkylation results by recovered H2PMo12O40-NMPIL@SiO2 are shown in Fig. 9. Results showed that the product yield in each run with no notable decline in catalytic efficiency, suggesting that the catalyst could be recovered and recycled up to six runs with no significant loss of catalytic activities. The recovered catalyst after six runs had no obvious change in the morphology and size, referring to the SEM image in comparison with fresh catalyst (Fig. 4j). Furthermore, XRD diffractogram of the catalyst after six runs revealed that H2PMo12O40-NMPIL@SiO2 preserved its original crystallinity during the reaction (Fig. 10).
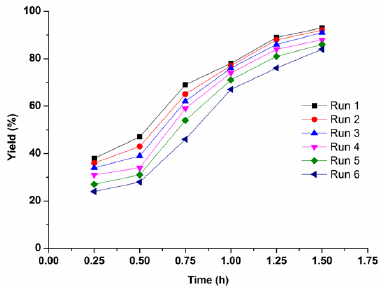
Fig. 9 Reusability of H2PMo12O40-NMPIL@SiO2 in Friedel-Crafts alkylation of indole with 2-phenyloxirane.
With the optimized reaction conditions in hand, a series of indoles and epoxides were tested to investigate the generality and scope of H2PMo12O40-NMPIL@SiO2. The alkylations of indole with various epoxides successfully proceeded to give the corresponding alcohols (Table 2, entries 1-6), whilst aliphatic oxiranes significantly decreased the reaction rate, and good yields were obtained after prolonged reaction time (Table 2, entries 5 and 6). On the other hand, other indoles such as 2-methyl-1H-indole, 5-methoxy-1H-indole and 5-nitro-1H-indole were also studied in the alkylation (Table 2, entries 7-9), good to excellent yields of the desired products were afforded. It was observed that the tested substituted indoles bearing electron-releasing groups furnished higher reaction rates than those with electron-withdrawing groups.
Table 2 H2PMo12O40-NMPIL@SiO2 catalyzed alkylation of indoles with epoxides. a
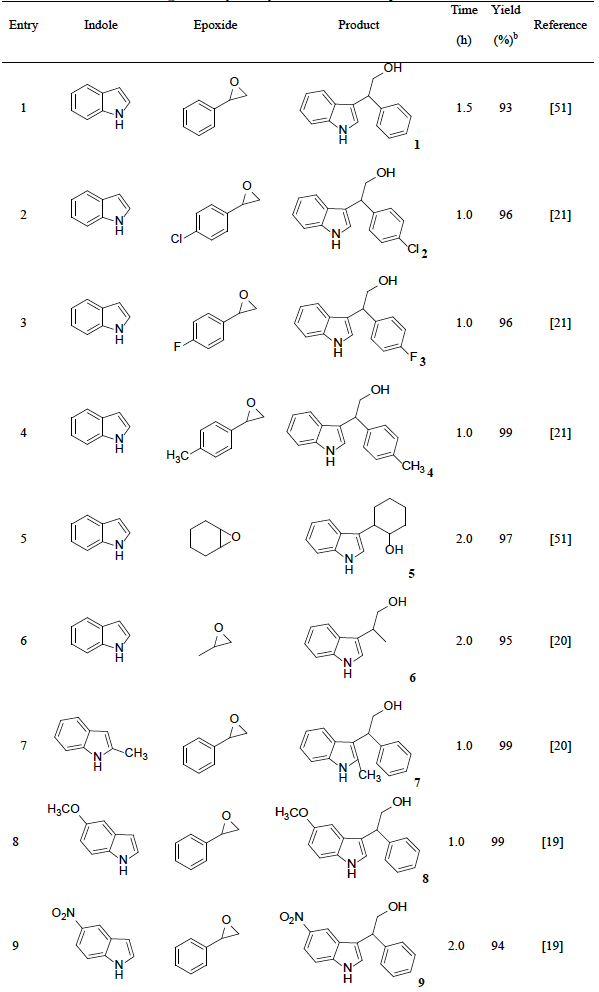
a Reaction conditions: indole (0.1 mol), epoxide (0.1 mol), CH2Cl2 (20 mL), H2PMo12O40-NMPIL@SiO2 (1.2 mol%), rt.
b Isolated yield.
To expand the scope of catalyst’s application, the alkylation of indoles with various alcohols were investigated. It was founded that the suitable reaction conditions were similar as the alkylation of indoles with epoxides. Initial experiments with using indole and benzyl alcohol were performed to establish the optimum conditions. As expected, CH2Cl2 was the efficient solvent and the effect of catalysts were also examined, the best result was the use of catalyst H2PMo12O40-NMPIL@SiO2 (Fig. 11). Next, we evaluated the different amount of H2PMo12O40-NMPIL@SiO2 for the alkylation, and it was found that 3.0 mol % of catalyst gave the best product yield and selectivity at room temperature for 2.5 h (Fig. 12). Therefore, the catalytic activities of H2PMo12O40-NMPIL@SiO2 for various substrates were assessed and the results are shown in Table 3. A variety of indoles were reacted with alcohols, which gave the desired products in good to excellent yields (Table 3, entries 1-10). It was found that indoles containing electron-releasing groups were more reactive than those with electron-withdrawing groups. As expected, the best yields were obtained with aryl alcohols (Table 3, entries 1-6 and 8-10). In the case of cyclohexanol, good yield of the product was obtained with longer reaction time (Table 3, entry7).
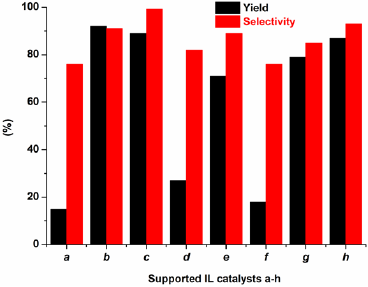
Fig. 11 Catalytic activities of various supported ILs in alkylation of indole with benzyl alcohol. Reaction conditions: indole (0.1 mol), benzyl alcohol (0.1 mol), CH2Cl2 (20 mL), catalyst (3.0 mol%) at room temperature for 2.5 h.
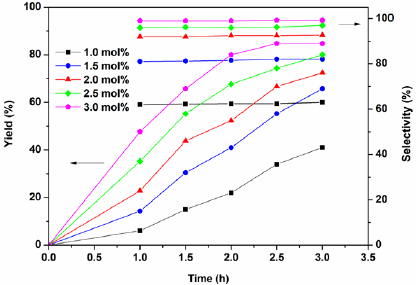
Fig. 12 Influence of catalyst amount on the alkylation of indole with benzyl alcohol. Reaction conditions: indole (0.1 mol), benzyl alcohol (0.1 mol), CH2Cl2 (20 mL), in different amounts of H2PMo12O40-NMPIL@SiO2 at room temperature.
Table 3 H2PMo12O40-NMPIL@SiO2 catalyzed alkylation of indoles with alcohols. a
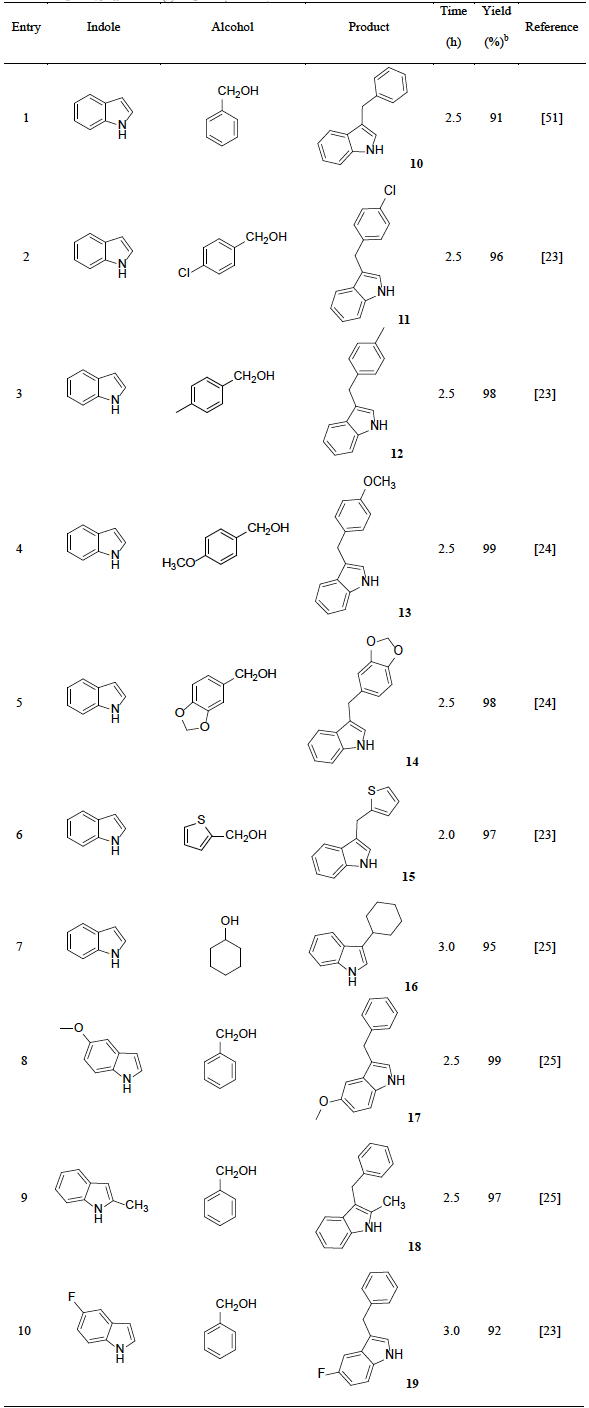
a Reaction conditions: indole (0.1 mol), alcohol (0.1 mol), CH2Cl2 (20 mL), H2PMo12O40-NMPIL@SiO2 (3 mol%), rt.
b Isolated yield.
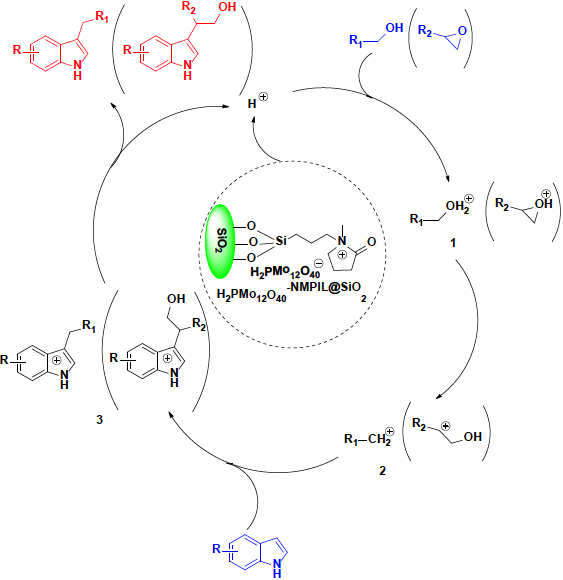
From these results, a possible reaction mechanism of the alkylation reaction of indoles with epoxides and alcohols in the presence of H2PMo12O40-NMPIL@SiO2 was proposed and depicted in Scheme 3 based on the previous literatures [18-25]. In this alkylation, the catalyst H2PMo12O40-NMPIL@SiO2 provides a source of H (+). At first, the substrate epoxide or alcohol was activated by H2PMo12O40-NMPIL@SiO2 to form the complex 1 and then was protonated to generate a stable cation 2, which reacted with indole to produce complex 3 via its nucleophilic attack. Subsequent elimination of proton (H+) to afford the desired product.
Conclusion
In conclusion, we have developed a novel and highly efficient chemical process for the preparation of 3-alkylindoles by alkylation of indoles catalyzed by supported functionalized ionic liquid H2PMo12O40-NMPIL@SiO2 at room temperature. Various indoles were reacted with epoxides or alcohols to give the corresponding 3-alkylindoles in good to excellent yields with high selectivities under mild conditions. This process was endowed with some attractive features, such as short reaction times, high yields, simple experimental procedure, good generality, easy recoverability and recyclability of catalyst. The above study would provide a green and highly efficient chemistry technology towards the preparation of 3-alkylindoles.
General
The reagents and solvents were purchased from Aldrich. Powder X-ray diffraction (XRD) patterns were collected on a Rigaku Ultima IV diffractometer. Scanning electron microscopy (SEM) and Energy dispersive X-ray (EDX) analysis were carried out on a JSM-7500F Scanning Electron Microscope. FTIR spectra were recorded on a Nicolet Nexus 470 Fourier transform infrared spectrometer. UV-Vis spectra were recorded on a Shimadzu UV-2450 spectrometer. Thermogravimetric analysis (TG) were measured on a Netzsch Thermoanalyzer STA 449. HPLC experiments were performed on a Shimadzu SCL-10A chromatograph. NMR spectra were recorded in CDCl3 at room temperature on a Bruker 400 MHz spectrometer. HRMS measurements were performed on a Waters Synapt spectrometer. Elemental analysis were performed on a Vario EL III instrument.
Preparation of supported ILs anion-NMPIL@SiO 2
Preparation of 1 Cl-NMPIL [44]: 1-methylpyrrolidin-2-one (99 g, 1 mol), (3-chloropropyl) triethoxysilane (241 g, 1 mol), toluene (700 mL) were refluxed for 72 h, and then the solvent was evaporated to give Cl-NMPIL. Preparation of 2 anion-NMPIL [45-48]: Cl-NMPIL (17 g, 0.05 mol) was dispersed in CH2Cl2 / CH3CN (120 mL) and stirred vigorously with LiNTf2, NaBF4 or KPF6 (0.06 mol) / ZnCl2 or AlCl3 (0.05 mol) for 24 h under reflux conditions. After cooled down to room temperature, the resulting material was isolated by filtration and evaporated under vacuum to give NTf2-NMPIL, BF4-NMPIL, PF6-NMPIL, ZnCl3-NMPIL or AlCl4-NMPIL; Cl-NMPIL (0.05 mol), H2SO4, H3PMo12O40 or CF3SO3H (0.05 mol) were stirred vigorously at 50 °C for 12 h, then the resulting material was dried under vacuum to give HSO4-NMPIL, H2PMo12O40-NMPIL or CF3SO3-NMPIL. Preparation of 3 anion-NMPIL@SiO2 [40-43,47]: anion-NMPIL (0.01 mol), activated silica (40 g), and toluene (500 mL) were stirred under reflux conditions for 24 h, the mixture was filtrated and washed with CH2Cl2 (3×20 mL), and dried under vacuum to afford the supported ILs anion-NMPIL@SiO2.
Preparation of 3-alkylindoles
A mixture of indole (0.1 mol), alcohol or epoxide (0.1 mol), CH2Cl2 (20 mL), and H2PMo12O40-NMPIL@SiO2 (1.2 mol% or 3.0 mol %) was stirred at room temperature for the desired time. The reaction progress was monitored by TLC and HPLC. Upon completion, the catalyst was filtered off and washed with ethanol (20 mL) and dried under vacuum. The filtrate was concentrated under reduced pressure and the crude product was purified by silica gel chromatography using ethyl acetate/petroleum ether cyclohexane as eluents to give the pure product. Fresh substrates were then recharged to the recovered catalyst and then recycled under identical reaction conditions. All products were identified by comparing their physical and HPLC spectra with those of commercial materials [19-21, 23-25, 51]. Spectroscopic data for products are as follows.
2-(1H-indol-3-yl)-2-phenylethanol (1). IR (KBr): v (cm-1); 3532, 3390, 3025, 2933, 1617, 1595, 1547, 1492, 1451, 1337, 1095, 1037, 748. 1H NMR: δ = 1.90 (br, 1H, OH), 3.98 (m, 2H, CH2), 4.25 (t, 1H, J = 6.7 Hz, CH), 6.91-7.43 (m, 8H, Ar-H), 7.43-7.54 (m, 2H, Ar-H), 8.13 (br, 1H, NH) ppm; 13C NMR: δ = 43.8, 73.2, 111.4, 115.2, 118.6, 119.7, 121.9, 124.5, 126.3, 128.1, 129.0, 136.4, 139.6, 140.3 ppm. HRMS m/z calcd for C16H15NO [M+H]+: 238.10, found 238.12. Anal. Calcd for C16H15NO: C, 80.95; H, 6.34; N, 5.86; O, 6.71, found C, 80.98; H, 6.37; N, 5.90; O, 6.74.
2-(4-Chlorophenyl)-2-(1H-indol-3-yl)ethanol (2). IR (KBr): v (cm-1); 3532, 3396, 3058, 2927, 1619, 1587, 1564, 1485, 1458, 1302, 1090, 1022, 758. 1H NMR: δ = 2.32 (br, 1H, OH), 4.03 (m, 2H, CH2), 4.31 (t, 1H, J = 6.9 Hz, CH), 6.89-7.41 (m, 7H, Ar-H), 7.47-7.56 (m, 2H, Ar-H), 8.13 (br, 1H, NH) ppm; 13C NMR: δ = 44.2, 73.3, 111.7, 115.3, 119.1, 119.8, 122.2, 124.6, 128.9, 129.3, 132.1, 136.7, 138.6, 140.2 ppm. HRMS m/z calcd for C16H14ClNO [M+H]+: 272.20, found 272.08. Anal. Calcd for C16H14ClNO: C, 70.68; H, 5.14; Cl, 13.02; N, 5.11; O, 5.86, found C, 70.72; H, 5.19; Cl, 13.05; N, 5.15; O, 5.89.
2-(4-fluorophenyl)-2-(1H-indol-3-yl)ethanol (3). IR (KBr): v (cm-1); 3542, 3410, 3057, 2881, 1617, 1548, 1490, 1455, 1417, 1342, 1251, 1093, 1045, 1015, 751. 1H NMR: δ = 2.45 (br, 1H, OH), 4.07 (m, 2H, CH2), 4.33 (t, 1H, J = 6.9 Hz, CH), 6.91-7.38 (m, 7H, Ar-H), 7.48-7.59 (m, 2H, Ar-H), 8.24 (br, 1H, NH) ppm; 13C NMR: δ = 44.7, 73.8, 111.6, 115.7, 116.3, 119.4, 120.3, 122.1, 124.8, 129.5, 135.3, 136.8, 140.1, 159.8 ppm. HRMS m/z calcd for C16H14FNO [M+H]+: 256.20, found 256.11. Anal. Calcd for C16H14FNO: C, 75.24; H, 5.51; F, 7.40; N, 5.45; O, 6.23, found C, 75.28; H, 5.53; F, 7.44; N, 5.49; O, 6.27.
2-(1H-indol-3-yl)-2-(p-tolyl)ethanol (4). IR (KBr): v (cm-1); 3520, 3382, 3016, 2937, 1625, 1598, 1554, 1497, 1446, 1320, 1087, 1032, 752. 1H NMR: δ = 1.92 (br, 1H, OH), 2.26 (s, 3H, CH3), 3.99 (m, 2H, CH2), 4.27 (t, 1H, J = 7.1 Hz, CH), 6.87-6.95 (m, 2H, Ar-H), 7.02-7.07 (m, 1H, Ar-H), 7.21-7.32 (m, 4H, Ar-H), 7.46-7.54 (m, 2H, Ar-H), 8.16 (br, 1H, NH) ppm; 13C NMR: δ = 21.2, 42.7, 73.6, 111.6, 114.9, 118.5, 120.3, 121.6, 124.7, 127.8, 129.4, 135.8, 136.7, 137.6, 140.1 ppm. HRMS m/z calcd for C17H17NO [M+H]+: 252.20, found 252.13. Anal. Calcd for C17H17NO: C, 81.21; H, 6.79; N, 5.54; O, 6.34, found C, 81.24; H, 6.82; N, 5.57; O, 6.37.
2-(1H-indol-3-yl)cyclohexanol (5). IR (KBr): v (cm-1); 3427, 2945, 1603, 1587, 1492, 1405, 1260, 1156, 1048, 742. 1H NMR: δ = 1.41-1.73 (m, 8H, CH2CH2CH2CH2), 1.95 (br, 1H, OH), 2.78 (m, 1H, CH), 3.61 (m, 1H, CH), 7.03-7.48 (m, 5H, Ar-H), 8.26 (br, 1H, NH) ppm; 13C NMR: δ = 23.8, 25.4, 33.8, 35.2, 51.1, 74.7, 111.3, 114.2, 118.6, 120.1, 121.9, 123.5, 129.1, 137.2 ppm. HRMS m/z calcd for C14H17NO [M+H]+: 216.10, found 216.13. Anal. Calcd for C14H17NO: C, 78.06; H, 7.93; N, 6.48; O, 7.41, found C, 78.10; H, 7.96; N, 6.51; O, 7.43.
2-(1H-indol-3-yl)propan-1-ol (6). IR (KBr): v (cm-1); 3435, 3008, 2942, 1613, 1579, 1496, 1412, 1347, 1273, 1251, 1078, 745. 1H NMR: δ = 1.27 (d, 2H, J = 7.1 Hz, CH2), 1.96 (br, 1H, OH), 3.06 (m, 1H, CH), 3.65 (m, 2H, CH2), 7.01-7.46 (m, 5H, Ar-H), 8.21 (br, 1H, NH) ppm; 13C NMR: δ = 18.7, 41.5, 70.4, 111.5, 113.9, 119.0, 119.7, 121.6, 123.3, 128.8, 136.9 ppm. HRMS m/z calcd for C11H13NO [M+H]+: 176.10, found 176.11. Anal. Calcd for C11H13NO: C, 75.37; H, 7.45; N, 7.96; O, 9.09, found C, 75.40; H, 7.48; N, 7.99; O, 9.13.
2-(2-Methyl-1H-indol-3-yl)-2-phenylethanol (7). IR (KBr): v (cm-1); 3530, 3395, 3054, 2928, 1620, 1595, 1562, 1492, 1457, 1312, 1095, 1024, 748. 1H NMR: δ = 2.01 (br, 1H, OH), 2.41 (s, 3H, CH3), 4.06 (m, 2H, CH2), 4.31 (t, 1H, J = 7.5 Hz, CH), 6.86-7.32 (m, 8H, Ar-H), 7.49-7.53 (m, 1H, Ar-H), 8.12 (br, 1H, NH) ppm; 13C NMR: δ = 12.8, 42.5, 73.4, 111.5, 114.2, 118.7, 120.1, 121.9, 126.2, 127.4, 128.1, 131.1, 136.5, 140.3 ppm. HRMS m/z calcd for C17H17NO [M+H]+: 252.20, found 252.13. Anal. Calcd for C17H17NO: C, 81.20; H, 6.78; N, 5.53; O, 6.35, found C, 81.24; H, 6.82; N, 5.57; O, 6.37.
2-(5-Methoxy-1H-indol-3-yl)-2-phenylethanol (8). IR (KBr): v (cm-1); 3385, 3051, 2925, 2854, 1612, 1572, 1435, 1297, 1126, 1092, 887, 746. 1H NMR: δ = 2.18 (br, 1H, OH), 3.87 (s, 3H, OCH3), 4.07 (m, 2H, CH2), 4.33 (t, 1H, J = 7.2 Hz, CH), 6.78-7.37 (m, 7H, Ar-H), 7.42-7.55 (m, 2H, Ar-H), 8.27 (br, 1H, NH) ppm; 13C NMR: δ = 43.6, 56.4, 73.7, 111.7, 114.5, 119.2, 121.7, 124.3, 126.3, 126.8, 127.9, 128.5, 140.5, 141.7, 153.8 ppm. HRMS m/z calcd for C17H17NO2 [M+H]+: 268.20, found 268.13. Anal. Calcd for C17H17NO2: C, 76.34; H, 6.36; N, 5.22; O, 11.95, found C, 76.38; H, 6.41; N, 5.24; O, 11.97.
2-(5-Nitro-1H-indol-3-yl)-2-phenylethanol (9). IR (KBr): v (cm-1); 3368, 3042, 2932, 2861, 1619, 1583, 1551, 1442, 1305, 1292, 1127, 1087, 753. 1H NMR: δ = 2.12 (br, 1H, OH), 4.03 (m, 2H, CH2), 4.27 (t, 1H, J = 7.5 Hz, CH), 7.14-7.73 (m, 7H, Ar-H), 8.12-8.26 (m, 2H, Ar-H), 8.38 (br, 1H, NH) ppm; 13C NMR: δ = 44.9, 74.2, 112.3, 114.2, 115.7, 124.6, 126.1, 126.9, 127.8, 128.6, 129.1, 132.5, 140.7, 142.3 ppm. HRMS m/z calcd for C16H14N2O3 [M+H]+: 283.10, found 283.10. Anal. Calcd for C16H14N2O3: C, 68.05; H, 4.97; N, 9.89; O, 16.96, found C, C, 68.07; H, 5.00; N, 9.92; O, 17.00.
3-Benzyl-1H-indole (10). IR (KBr): v (cm-1); 3361, 3023, 2827, 1609, 1563, 1428, 1015, 893, 764. 1H NMR: δ = 4.03 (s, 2H, CH2), 6.97-7.43 (m, 9H, Ar-H), 7.52 (m, 1H, Ar-H), 8.09 (br, 1H, NH) ppm; 13C NMR: δ = 30.6, 111.3, 113.4, 119.3, 119.9, 121.8, 123.6, 126.1, 127.1, 128.3, 129.7, 136.6, 137.2 ppm. HRMS m/z calcd for C15H13N [M+H]+: 208.20, found 208.11. Anal. Calcd for C15H13N: C, 86.88; H, 6.30; N, 6.73, found C, 86.92; H, 6.32; N, 6.76.
3-(4-Chlorobenzyl)-1H-indole (11). IR (KBr): v (cm-1); 3369, 3115, 2901, 1625, 1512, 1427, 1338, 1078, 752, 723, 695. 1H NMR: δ = 4.15 (s, 2H, CH2), 6.95-7.49 (m, 8H, Ar-H), 7.63 (m, 1H, Ar-H), 8.17 (br, 1H, NH) ppm; 13C NMR: δ = 31.3, 111.6, 112.9, 119.5, 120.4, 121.3, 123.8, 127.8, 129.2, 131.6, 132.5, 135.1, 137.8 ppm. HRMS m/z calcd for C15H12ClN [M+H]+: 242.10, found 242.07. Anal. Calcd for C15H12ClN: C, 74.50; H, 4.95; Cl, 14.65; N, 5.76, found C, 74.53; H, 5.00; Cl, 14.67; N, 5.79.
3-(4-Methylbenzyl)-1H-indole (12). IR (KBr): v (cm-1); 3517, 3378, 3025, 2943, 1584, 1465, 1432, 1376, 1056, 1013, 765. 1H NMR: δ = 2.38 (s, 3H, CH3), 4.02 (s, 2H, CH2), 6.91-7.42 (m, 8H, Ar-H), 7.56 (m, 1H, Ar-H), 7.97 (br, 1H, NH) ppm; 13C NMR: δ = 22.1, 31.3, 111.2, 113.0, 119.2, 120.1, 121.9, 123.3, 127.7, 128.8, 129.7, 133.5, 136.2, 136.9 ppm. HRMS m/z calcd for C16H15N [M+H]+: 222.10, found 222.12. Anal. Calcd for C16H15N: C, 86.82; H, 6.80; N, 6.29, found C, 86.84; H, 6.83; N, 6.33.
3-(4-Methoxybenzyl)-1H-indole (13). IR (KBr): v (cm-1); 3564, 3386, 3052, 2923, 1576, 1461, 1420, 1382, 1068, 1026, 761. 1H NMR: δ = 3.79 (s, 3H, CH3), 4.09 (s, 2H, CH2), 6.85-6.92 (m, 3H, Ar-H), 7.09-7.21 (m, 4H, Ar-H), 7.37-7.49 (m, 2H, Ar-H), 7.95 (br, 1H, NH) ppm; 13C NMR: δ = 31.6, 56.2, 111.5, 113.1, 114.6, 119.1, 119.8, 121.3, 122.8, 127.3, 128.7, 129.9, 136.1, 158.2 ppm. HRMS m/z calcd for C16H15NO [M+H]+: 238.20, found 238.12. Anal. Calcd for C16H15NO: C, 80.97; H, 6.35; N, 5.86; O, 6.71. Found: C, 80.98; H, 6.37; N, 5.90; O, 6.74.
3-(Benzo[d][1,3]dioxol-5-ylmethyl)-1H-indole (14). IR (KBr): v (cm-1); 3436, 2956, 1627, 1594, 1486, 1424, 1297, 1098, 1031, 759. 1H NMR: δ = 4.05 (s, 2H, CH2), 5.96 (s, 2H, CH2), 6.67-6.81 (m, 3H, Ar-H), 6.98-7.06 (m, 2H, Ar-H), 7.21-7.47 (m, 3H, Ar-H), 7.98 (br, 1H, NH) ppm; 13C NMR: δ = 31.7, 101.1, 108.2, 109.5, 111.3, 116.2, 119.4, 119.7, 121.6, 122.2, 122.6, 127.5, 135.4, 136.7, 145.9, 147.8 ppm. HRMS m/z calcd for C16H13NO2 [M+H]+: 252.10, found 252.10. Anal. Calcd for C16H13NO2: C, 76.43; H, 5.19; N, 5.54; O, 12.71. Found: C, 76.48; H, 5.21; N, 5.57; O, 12.73.
3-(Thiophen-2-ylmethyl)-1H-indole (15). IR (KBr): v (cm-1); 3343, 3112, 3053, 2836, 1636, 1523, 1467, 1421, 757, 693. 1H NMR: δ = 4.32 (s, 2H, CH2), 6.88-7.36 (m, 7H, Ar-H), 7.56 (m, 1H, Ar-H), 7.96 (br, 1H, NH) ppm; 13C NMR: δ = 26.4, 110.3, 111.5, 115.9, 119.4, 119.9, 122.5, 122.7, 123.8, 125.2, 127.1, 136.9, 145.2 ppm. HRMS m/z calcd for C13H11NS [M+H]+: 214.10, found 214.06. Anal. Calcd for C13H11NS: C, 73.17; H, 5.16; N, 6.54; S, 15.01, found C, 73.20; H, 5.20; N, 6.57; S, 15.03.
3-Cyclohexyl-1H-indole (16). IR (KBr): v (cm-1); 3436, 2932, 1625, 1593, 1476, 1413, 1295, 1096, 1054, 761. 1H NMR: δ = 1.42-1.81 (m, 10H, CH2CH2CH2CH2CH2), 2.74 (m, 1H, CH), 7.01-7.52 (m, 5H, Ar-H), 8.02 (br, 1H, NH) ppm; 13C NMR: δ = 25.2, 26.1, 35.5, 43.7, 111.2, 113.9, 118.8, 119.7, 121.3, 123.2, 128.9, 136.9 ppm. HRMS m/z calcd for C14H17N [M+H]+: 200.20, found 200.14. Anal. Calcd for C14H17N: C, 84.34; H, 8.57; N, 7.01, found C, 84.37; H, 8.60; N, 7.03.
3-Benzyl-5-methoxy-1H-indole (17). IR (KBr): v (cm-1); 3395, 3064, 2951, 2860, 1635, 1592, 1437, 1386, 1262, 1113, 1078, 753. 1H NMR: δ = 3.81 (s, 3H, OCH3), 4.06 (s, 2H, CH2), 6.86-6.94 (m, 3H, Ar-H), 7.18-7.41 (m, 6H, Ar-H), 7.92 (br, 1H, NH) ppm; 13C NMR: δ = 30.9, 55.6, 101.2, 112.1, 112.4, 113.8, 123.4, 125.6, 128.1, 128.9, 129.6, 136.5, 154.2 ppm. HRMS m/z calcd for C16H15NO [M+H]+: 238.20, found 238.12. Anal. Calcd for C16H15NO: C, 80.97; H, 6.35; N, 5.86; O, 6.72. Found: C, 80.98; H, 6.37; N, 5.90; O, 6.74.
3-Benzyl-2-methyl-1H-indole (18). IR (KBr): v (cm-1); 3542, 3387, 3036, 2938, 1631, 1578, 1484, 1436, 1382, 1321, 1078, 759. 1H NMR: δ = 2.35 (s, 3H, CH3), 4.07 (s, 2H, CH2), 6.95-7.12 (m, 3H, Ar-H), 7.22-7.38 (m, 6H, Ar-H), 7.73 (br, 1H, NH) ppm; 13C NMR: δ = 12.7, 32.6, 109.7, 111.3, 118.9, 119.6, 121.2, 125.4, 127.8, 128.4, 129.3, 131.6, 136.7 ppm. HRMS m/z calcd for C16H15NO [M+H]+: 222.10, found 222.12. Anal. Calcd for C16H15N: C, 86.82; H, 6.81; N, 6.29. Found: C, 86.84; H, 6.83; N, 6.33.
3-Benzyl-5-fluoro-1H-indole (19). IR (KBr): v (cm-1); 3526, 3023, 2892, 1615, 1532, 1497, 1422, 1347, 1276, 1180, 923, 896, 758, 694. 1H NMR: δ = 3.91 (s, 2H, CH2), 6.96-7.53 (m, 9H, Ar-H), 7.92 (br, 1H, NH) ppm; 13C NMR: δ = 31.5, 109.3, 112.3, 112.9, 115.4, 122.8, 126.1, 128.8, 129.2, 129.6, 132.4, 136.7, 158.2 ppm. HRMS m/z calcd for C15H12FN [M+H]+: 226.10, found 226.10. Anal. Calcd for C15H12FN: C, 79.95; H, 5.34; F, 8.41; N, 6.18, found C, 79.98; H, 5.37; F, 8.43; N, 6.22.




![One-pot, three-component Synthesis of pyrrolo[2,3-d]pyrimidine Derivatives](/img/en/prev.gif)






 text new page (beta)
text new page (beta)