Introduction
In order to construct highly functionalized small organic molecules from readily available starting materials in one pot with inherent flexibility, the multicomponent reactions can effectively create molecular complexity and diversity and minimize time, labor, cost, and waste products [1]. Therefore, many organic chemists have focused their attention on multicomponent reactions in the synthesis of heterocyclic and bioactive molecules.
In natural products, xanthene and its derivatives are an important class of oxygen-containing heterocyclic compounds that possess biological activities [2]. Xanthene and its derivatives have important biological and pharmacological activities such as antiviral [3], anti-inflammatory [4], antibacterial [5], antimalarial [6], antitumor [7], and antiplasmodial [8] activities. In addition, they can be used in photodynamic therapy [9] and as an antagonist; they inhibit the paralyzing action of zoxazolamine [10]. Furthermore, due to their useful spectroscopic properties, they are used as dyes [11]. pH-sensitive fluorescent materials for visualization of biomolecular assemblies [12], and in laser technologies [13]. Fig. 1 presents various methods that have been reported in the literature for the synthesis of xanthene derivatives.
However, most of these methods have the following limitations: use of expensive reagents, low yields or a mixture of products, long reaction time, strongly acidic conditions, use of excess reagents and catalysts, use of a toxic organic solvent, and drastic reaction conditions. Therefore, for the preparation of xanthene derivatives under neutral mild and practical conditions, it is essential to search a new and efficient catalyst with high catalytic activity, short reaction time, recyclability, and simple reaction conditions. The current reaction procedure can be enhanced by facilitating the reaction between 2-naphthol and alkyl/aryl aldehydes by employing various catalysts such as Bronsted acids [25, 27], solid-supported reagent [28, 29], metal salt [30, 31], metal triflate [32], I2 [33], phosphosulfonic acid [34], 1,3,5 trichloro-2,4,6-triazinetrion [35], vanadate sulfuric acid [36], HBF4-SiO2 [37], and SelectfluorTM [38] . However, the improvised method may also have the same limitations such as an expensive and toxic catalyst, acidic condition, long reaction time, low yield, the preparation of catalyst required in some cases, and the use of heavy and toxic metals. Hence, it is imperative to find a new and efficient catalyst with high catalytic activity, short reaction time, inexpensive and easy availability, and simple and feasible procedure for the preparation of xanthene derivatives.
In recent years, much attention has been focused on developing more economical and environment-friendly conversion processes. CaCl2 is an inexpensive and commercially available reagent that has been recently shown to be a very good catalyst in organic reactions [39-42]. This study reports an efficient, practical, environmentally benign, and high yielding method for the synthesis of xanthene using CaCl2 as a catalyst.
RESULTS AND DISCUSSION
We have reported earlier a highly efficient synthetic route of flavanone, chalcone and dihydropyrimidones under microwave conditions [43-45] for the development of green organic transformation [46-47]. In this study, we report a simple and efficient method for xanthene synthesis under microwave condition that results in high yield and no by-products. First, we studied the condensation reaction between β-naphthol (2 mmol) (1a) and benzaldehyde (1 mmol) in the presence of calcium chloride/HCl as a co-catalytic system. β-Naphthol and benzaldehyde dissolved in dichloromethane were added to calcium chloride/HCl; they can be adsorbed after evaporating dichloromethane to give a free-flowing powder. Concentrated HCl increases the acidic character of calcium chloride and the reaction rate. The resulting powder was exposed to microwave (400 W; power level: 2). The progress of the reaction was monitored by TLC using using ethyl acetate: pet ether (2:8; 60-80◦C) at intervals of 1 min. After 12 min, it was observed that the reaction proceeded in the forward direction and formed a new product. The reaction workup followed by the recrystallization of the crude product from ethanol resulted in the pure product. The structure of the product was confirmed by spectral data. In the 1H NMR and 13C NMR spectra, the aliphatic CH proton of 14-(2-phenyl)-14H-dibenzo[a,j]xanthene (3a) and CH carbon appeared as a singlet at 7.48 ppm and 32.4 ppm, respectively, which was in agreement with the reported values for 14-(2-phenyl)-14H-dibenzo[a,j]xanthene. The aliphatic CH proton was obtained as singlet in all the compounds. Subsequently, to optimize the reaction conditions, varying concentrations of calcium chloride (5, 10, 20 and 40 mol%) were used as 1:1 and 1:2 catalyst. The 5 mol% catalyst gave the maximum yield in a short time interval. The condensation reaction did not take place in the absence of calcium chloride, indicating that a catalyst is necessary for the condensation reaction even under microwave condition.
To examine the applicability of the optimized reaction condition for the synthesis of different dibenzo[a,j]xanthene derivatives, the reaction between β-naphthol and substituted Benzaldehyde (2a-t) was examined (Fig. 2). A smooth one-pot conversion produced a series of corresponding dibenzo[a,j]xanthene (3a-t) (Table 1).
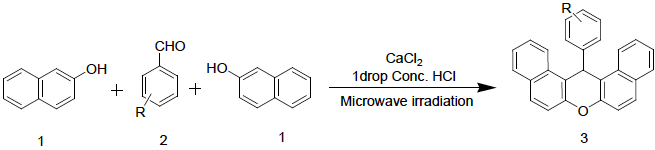
Fig. 2 Synthesis of Xanthene via one-pot condensation between 2-naphthol and benzaldehyde catalyzed by calcium chloride/Conc. HCl under microwave condition
Table 1 Synthesis of aryl 14H-dibenzo[a,j] xanthene derivatives in the presence of calcium chloride/Conc.HCl as a catalyst from β-naphthol and aromatic aldehydes under microwave conditiona
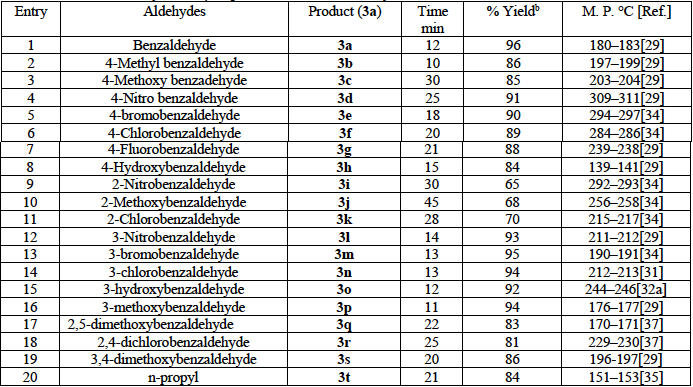
a: Reaction Conditions: 2 mmole of β-naphthol, 1 mmole substituted benzaldehyde, calcium chloride (0.5 mmol)/ 1 drop conc.HCl irradiated with microwave; b: isolated yield after purification
It was hypothesized that the products were obtained via the formation of a Knoevenagel product followed by Michael addition. One molecule of β-naphthol was condensed with aldehyde, which was activated by calcium chloride to form the intermediate Knoevenagel product. Subsequently, the active methylene of the second molecule of β-naphthol reacted with the Knoevenagel product via conjugate Michael addition to generate the intermediate, which underwent intramolecular cyclodehydration to give the desired product.
To evaluate the improvement by the use of calcium chloride/HCl over other reported catalysts, the pseudo three-component coupling reaction of β-naphthol and benzaldehyde was used as a representative example (Table 2). However, in most cases, the comparative yield of the desired product obtained with the CaCl2/HCl-catalyzed procedures required short reaction times compared to entry 1, 2, 4 and 5, 7, 9, 12, 13, 14 or low catalyst loading with respect to entry 1, 3, 9, 15 and some methods require high temperature entry 1, 3, 4, 5, 6, 11, 12, 13, 14, 15. These results clearly demonstrate that CaCl2/HCl is an equal or more efficient catalyst for this three-component reaction.
Experimental
The reagents were purchased from Loba, Merck, SRL, and Signa Aldrich and Spectrochem and used without further purification. The melting points were recorded by the open capillary method and were uncorrected. The reactions were irradiated in a microwave oven (Onidia India Ltd.). 1HNMR and 13CNMR spectra were obtained in CDCl3 on a Bruker AV-300 (300 MHz) spectrometer using TMS as an internal standard. The IR spectra were recorded on a Nicolet Fourier Transform spectrometer. TLC was performed on GF-25U (Anal. Tech) plates and silica gel glass-backed plates.
General procedure for the preparation of aryl 14h-dibenzo[a,j] xanthene derivatives: A mixture of β-naphthol (2 mmol) and substituted benzaldehyde (1 mmol) was dissolved in 5mL dichloromethane. This solution was added to calcium chloride (0.1 mmol) and one drop of concentrated HCl was added and well swirled in a 50-mL beaker. The solvent was removed under reduced pressure using a rotatory evaporator. The resulting free-flowing powder was taken in a 20mL beaker and irradiated in a microwave oven at 400 W (Onidia India Ltd.). The reaction mixture was irradiated for a specified time (see Table 1). The progress of the reaction was monitored by TLC. Upon completion of the reaction, the reaction mixture was diluted with cold water. The resulting solid product was filtered using a suction pump, washed with cold ethanol, and dried. The product was purified by crystallization method (EtOH). All the products are known in the literature and were characterized by comparison of IR and NMR spectra with authentic samples.
Spectral data of synthesized compounds
14-phenyl-14H-dibenzo[a, j]xanthene 3a: Pale yellow solid, IR (KBr, cm-1) : νmax 3072, 2946, 1592, 1456 cm-1; 1HNMR (300MHz, CDCl3): δ 6.47(s, 1H, CH), 8.35-6.98 (m, 17H, ArH); 13CNMR (75MHz, CDCl3): δ 38.2, 117.2, 118.3, 122.9, 124.4, 126.5, 126.9, 128.4, 128.8, 129.2, 131.3, 131.7, 145.3, 149.2; ESI-MS(m/z): 348.41[M]+.
14-(4-methylphenyl)-14H-dibenzo[a, j]xanthene 3b: Yellow solid, IR (KBr, cm-1) : νmax 3076, 2905, 1618, 1589 cm-1; 1HNMR (300MHz, CDCl3): δ 2.15 (s, 3H, CH3), 6.45(s, 1H, CH), 8.37-6.94 (m, 16H, ArH); 13CNMR (75MHz, CDCl3): δ 21.2, 37.4, 117.3, 118.3, 122.9, 124.3, 126.8, 128.2, 128.9, 129.1, 129.4, 131.4, 131.6, 136.2, 142.3, 148.9; ESI-MS(m/z): 372.11[M] +.
14-(4-methoxyphenyl)-14H-dibenzo[a, j]xanthene 3c: pink solid, IR(KBr, cm-1) : νmax 3057, 2920, 1583, 1432 cm-1; 1HNMR (300MHz, CDCl3): δ 3.72 (s, 3H, OCH3), 6.43(s, 1H, CH), 8.34-6.56(m, 16H, ArH); 13CNMR (75MHz, CDCl3): δ 36.8, 55.4, 114.2, 117.4, 118.2, 122.4, 124.2, 126.9, 129.1, 129.9, 131.4, 132.0, 149.2, 158.3; ESI-MS(m/z): 388.40[M] +.
14-(4-nitrophenyl)-14H-dibenzo[a, j]xanthene 3d: yellow solid, IR(KBr, cm-1) : νmax 3092, 2956, 1620, 1435 cm-1; 1HNMR (300MHz, CDCl3): δ 6.61(s, 1H, CH), 8.42-6.82(m, 16H, ArH); 13CNMR (75MHz, CDCl3): δ 38.1, 115.3, 115.9, 118.1, 122.2, 124.0, 124.8, 127.3, 128.8, 129.8, 131.4, 146.7, 149.0, 152.5; ESI-MS(m/z): 403.12[M] +.
14-(4-bromophenyl)-14H-dibenzo[a, j]xanthene 3e: Pale yellow solid, IR(KBr, cm-1) : νmax 3025, 1626, 1583 cm-1; 1HNMR (300MHz, CDCl3): δ 6.48 (s, 1H, CH), 7.23-8.33(m, 16H, ArH); 13CNMR (75MHz, CDCl3): δ 37.1, 116.4, 117.9, 126.8, 128.4, 128.9, 129.4, 131.1, 131.7, 132.4, 143.3, 148.5; ESI-MS(m/z): 437.33[M] +.
14-(4-chlorophenyl)-14H-dibenzo[a, j]xanthene 3f: Brown solid, IR(KBr, cm-1) : νmax 3040, 2921, 1616, 1587 cm-1; 1HNMR (300MHz, CDCl3): δ 6.49(s, 1H, CH), 8.29-7.06 (m, 16H, ArH); 13CNMR (75MHz, CDCl3): δ 37.2, 116.6, 118.0, 126.8, 128.7, 129.1, 129.5, 131.0, 131.3, 132.2, 143.6, 148.7; ESI-MS(m/z): 392.82[M] +.
14-(4-fluorophenyl)-14H-dibenzo[a, j]xanthene 3g: yellow solid, IR(KBr, cm-1) : νmax 3025, 2907, 1623, 1594 cm-1; 1HNMR (300MHz, CDCl3): δ 6.50(s, 1H, CH), 8.31-7.10m(m, 16H, ArH); 13CNMR (75MHz, CDCl3): δ 37.3, 116.7, 118.2, 126.9, 128.8, 129.2, 129.6, 131.2, 131.4, 132.1, 143.8, 148.9; ESI-MS(m/z): 376.23[M] +.
14-(4-Hydroxyphenyl)-14H-dibenzo[a, j]xanthene 3h: pale organge solid, IR(KBr, cm-1) : νmax 3410, 3034, 2935, 1608, 1589 cm-1; 1HNMR (300MHz, CDCl3): δ 6.41 (s, 1H, CH), 8.35-6.54(m, 16H, ArH), 11.08 (s, 1H, OH); 13CNMR (75MHz, CDCl3): δ 36.9, 115.1, 117.7, 118.1, 122.6, 124.4, 126.7, 128.8, 129.1, 129.4, 131.7, 131.8, 137.7, 148.8, 153.9; ESI-MS(m/z): 374.13[M] +.
14-(2-nitrophenyl)-14H-dibenzo[a, j]xanthene 3i: Yellow solid, IR(KBr, cm-1) : νmax 3088, 2941, 1624, 1437; 1HNMR (300MHz, CDCl3): δ 6.67 (s, 1H, CH), 8.05-7.13(m, 16H, ArH); 13CNMR (75MHz, CDCl3): δ 39.5, 117.8, 118.2, 122.7, 125.0, 125.2, 127.7, 127.9, 128.8, 129.9, 131.3, 132.6, 134.4, 140.4, 147.3, 151.8; ESI-MS(m/z): 403.41[M] +.
14-(2-methoxyphenyl)-14H-dibenzo[a, j]xanthene 3j: Yellow solid, IR(KBr, cm-1) : νmax 3017, 2943, 1589, 1483; 1HNMR (300MHz, CDCl3): δ 3.67 (s, 3H, OCH3), 6.39(s, 1H, CH), 8.36-6.46(m, 16H, ArH); 13CNMR (75MHz, CDCl3): δ 37.3, 55.2, 116.2, 117.4, 117.9, 121.5, 124.8, 125.4, 127.8, 129.5, 131.1, 131.9, 134.3, 142.3, 146.8, 152.2; ESI-MS(m/z): 388.14[M] +.
14-(2-chlorophenyl)-14H-dibenzo[a, j]xanthene 3k: Brown solid, IR(KBr, cm-1) : νmax 3039, 1593, 1453,1413; 1HNMR (300MHz, CDCl3): δ 6.53 (s, 1H, CH), 8.37-7.03 (m, 16H, ArH); 13CNMR (75MHz, CDCl3): δ 37.4, 116.7, 117.3, 122.6, 123.4, 125.6, 126.6, 127.1, 128.1, 128.9, 129.3, 130.4, 131.1, 143.4, 147.8; ESI-MS(m/z): 392.10[M] +.
14-(3-nitrophenyl)-14H-dibenzo[a ,j]xanthene 3l: Yellow solid, IR(KBr, cm-1) : νmax 3083, 2923, 1589, 1521, 1428; 1HNMR (300MHz, CDCl3): δ 6.57 (s, 1H, CH), 8.39-7.21 (m, 16H, ArH); 13CNMR (75MHz, CDCl3): δ 39.2, 105.6, 115.6, 118.4, 119.7, 122.7, 123.4, 127.8, 128.4, 129.6, 130.5, 133.6, 137.8, 137.9, 153.4, 154.4; ESI-MS(m/z): 403.42[M] +.
14-(3-bromophenyl)-14H-dibenzo[a, j]xanthene 3m: Pale yellow solid, IR(KBr, cm-1) : νmax 3059, 1597, 1427,1408; 1HNMR (300MHz, CDCl3): δ 6.45(s, 1H, CH), 7.05-8.29(m,16H, ArH); 13CNMR (75MHz, CDCl3): δ 37.9, 116.6, 118.4, 122.7, 122.9, 124.6, 126.9, 127.4, 129.2, 129.3, 129.9, 130.2, 131.2, 131.4, 131.6, 147.3, 149.2; ESI-MS(m/z): 437.32[M] +.
14-(3-chlorophenyl)14H-dibenzo[a, j]xanthene 3n: Brown solid, IR(KBr, cm-1) : νmax 3049, 2923, 1617, 1587, 1430; 1HNMR (300MHz, CDCl3): δ 6.44 (s, 1H, CH), 6.93-8.25 (m, 16H, ArH); 13CNMR (75MHz, CDCl3): δ 37.9, 116.2, 118.0, 122.2, 124.4, 126.3, 126.7, 127.2, 128.3, 128.9, 129.2, 129.6, 131.1, 131.4, 134.7, 146.6; ESI-MS(m/z): 392.15[M] +.
14-(3-hydroxyphenyl)-14H-dibenzo[a, j]xanthene 3o: Pink solid, IR(KBr, cm-1) : νmax 3412, 1593, 1510, 1408; 1HNMR (300MHz, CDCl3): δ 6.44(s, 1H, CH), 6.53-8.33(m, 16H, ArH); 13CNMR (75MHz, CDCl3): 37.4, 115.3, 117.6, 118.2, 123.1, 124.6, 127.1, 128.9, 129.3, 129.8, 131.3, 131.7, 137.9, 149.2, 154.3; ESI-MS(m/z): 374.21[M] +.
14-(3-methoxyphenyl)-14H-dibenzo[a, j]xanthene 3p: Yellow solid, IR(KBr, cm-1) : νmax 3064, 3008, 2913, 15781432; 1HNMR (300MHz, CDCl3): δ 3.72(s, 3H, OCH3), 6.42(s, 1H, CH), 8.37-6.50(m, 16H, ArH); 13CNMR (75MHz, CDCl3): δ 37.1, 55.3, 116.1, 117.1, 118.0, 121.2, 124.9, 125.2, 127.9, 129.2, 131.2, 132.0, 134.5, 142.5, 146.9, 152.6; ESI-MS(m/z): 388.16[M] +.
14-(2,5-dimethoxyphenyl)-14-dibenzo[a, j]xanthene 3q: Yellow solid, IR(KBr, cm-1) : νmax 3017, 2930, 2827, 1619, 1589, 1445, 1247; 1HNMR (300MHz, CDCl3): δ 3.65 (s, 3H, OCH3), 3.68(s, 3H, OCH3), 6.41(s, 1H, CH), 8.41-6.86(m, 15H, ArH); 13CNMR (75MHz, CDCl3): δ 31.4, 55.2, 55.7, 111.3, 111.9, 117.3, 118.4, 123.8, 124.6, 128.9, 129.2, 130.1, 132.2, 135.8, 148.4, 148.9, 154.2; ESI-MS(m/z): 418.13[M] +.
14-(2,4-dichlorophenyl)-14-dibenzo[a, j]xanthene 3r: Brown solid, IR(KBr, cm-1) : νmax 3048, 2913, 1623, 1585, 1432; 1HNMR (300MHz, CDCl3): δ 6.64(s, 1H, CH), 6.91-8.34(m, 15H, ArH); 13CNMR (75MHz, CDCl3): δ 35.8, 117.2, 117.8, 124.2, 127.3, 128.2, 129.2, 129.3, 130.6, 131.0, 132.7, 132.9, 143.0, 148.9; ESI-MS(m/z): 427.13[M] +.
14-(3,4-dimethoxyphenyl)-14-dibenzo[a, j]xanthene 3s: Yellow solid, IR(KBr, cm-1) : νmax 3017, 2910, 1608, 1578, 1419, 1253; 1HNMR (300MHz, CDCl3): δ 3.69 (s, 3H, CH3), 3.74 (s, 3H, CH3), 6.46(s, 1H, CH), 6.89-8.33(m, 15H, ArH); 13CNMR (75MHz, CDCl3): δ 36.3, 55.2, 55.6, 109.9, 113.3, 117.4, 117.9, 121.0, 122.6, 124.2, 126.4, 128.6, 128.9, 131.6, 137.2, 144.3, 146.8, 148.9; ESI-MS(m/z): 418.17[M] +.
14-(n-propyl)-14-dibenzo[a, j]xanthene 3t: White solid, IR(KBr, cm-1) : νmax 3057, 2915, 1594, 1435; 1HNMR (300MHz, CDCl3): δ 1.08 (t, J= 7Hz, 3H, CH3), 1.32(m, 2H, CH2), 1.98(m, 2H, CH2), 4.20(t, J=7Hz, 1H, CH), 8.10-6.84(m, 12H, ArH); 13CNMR (75MHz, CDCl3): δ 15.2, 20.3, 42.4, 43.5, 115.2, 118.4, 122.2, 123.3, 126.7, 128.2, 128.8, 129.2, 133.9, 153.2; ESI-MS(m/z): 324.10[M] +.
Conclusion
In this study, we have developed an efficient, absolutely clean and high yielding eco-friendly method, for the synthesis of aryl 14H-dibenzo[a,j] xanthenes derivatives under microwave conditions using calcium chloride as an ionic solid catalyst. The merits of this method are high yield, feasibility, short reaction time, minimal environmental impact, and non-use of toxic solvents.This makes it one of the attractive and practical protocols for the synthesis of aryl 14H-dibenzo[a,j] xanthene derivatives.





![One-pot, three-component Synthesis of pyrrolo[2,3-d]pyrimidine Derivatives](/img/es/next.gif)





 nueva página del texto (beta)
nueva página del texto (beta)




