Introduction
Olefin metathesis, as a technology that allows direct modification of the double bonds of unsaturated natural resources has enormous potential in the transition from petrochemicals to bio-renewable feedstocks [1]. In this perspective, the availability of robust and active catalysts has enabled the metathesis transformations of challenging substrates. The cross-metathesis of natural rubber, butadiene-styrene copolymer, and trans-polyisoprene with ethylene (ethenolysis), monoterpenes, triglycerides from plant oils, and functionalized olefins using ruthenium-based catalysts has been carried out resulting in an efficient synthesis of several telechelic oligomers [2-12].
The chicozapote, sapodilla or chicle tree (Manilkara zapota) is one of the species of genus Manilkara belonging to the Sapotaceae family and the Magnoliopsida class. Chemical studies showed the presence of polysaccharides, triterpenes, alkaloids, saponins, tannins, and flavonoids which highlighted the great biological potential of the genus [13-16]. Chicle from the original Maya word sicté [17], has been used for many purposes, such as plant-based drugs in traditional medicine [18], fruit production, and the extraction of the latex sap for use in chewing gum and for the manufacture of adhesives, paints, and varnishes resistant to water, and for insulation in electric conduction cables [19-20]. In addition to being used for seedling [21], the seeds have been considered for biodiesel production due to their oil content of 23-30% [22].
The sapodilla is a fairly slow-growing, long-lived small to medium evergreen tree, rich in white, gummy latex. This tropical fruit tree is native to Mexico and Central America [18]. It is also cultivated on a large scale in India, Thailand, Cambodia, Malaysia, Indonesia and, Bangladesh mainly for its fruit. In Mexico, the sapodilla is present primarily on the Gulf and Pacific coasts, Chiapas, the Yucatan peninsula and the Great Peten region neighboring Belize and Guatemala, where 1,500 ha are devoted to fruit production (mainly in the states of Campeche and Veracruz), while 4,000 ha are grown primarily for chicle and it is common to find up to 50 adult wild trees/ha [20, 23- 24]. The states of Campeche and Quintana Roo are the main producers of certified organic chewing gum, which main demand is from Asia and Italy. The preference for chicle over other sources of natural gum and cheaper synthetic resins is explained by the texture, elasticity, and capacity of chicle to absorb flavors [24].
The trees frequently grow from 15 to 30 m high and a meter and a half diameter, have a dense wood and take about eight years to mature sufficiently to be tapped for their latex, which is a mixture of terpene-based molecules, such as cis and trans-polyisoprene and pentacyclic triterpenes [17, 25 - 29]. The quantity of latex that can be derived from a single tree also varies widely, from about three kg to as much as 15 kg. The trees are tapped during the rainy season (August through January) by itinerant tappers, called chicleros, who locate chicle trees, make zigzag cuts in the bark, and collect the latex exuded. The trees normally bleed for a maximum of 20 h after tapping incisions are made and are tapped every three or four years [30]. The latex is boiled in an open vessel with constant stirring to reduce the water content to below 40% obtaining a sticky and elastic consistency. The concentrated latex is then poured into molds to form, on cooling, brown-colored blocks of about eight to 12 kg each [26]. Since the harvest of trees for timber is prohibited in major tapping regions and the selective extraction of this resource does not affect the diversity of the forest, the harvesting of chicle could play a role in the conservation of the tropical forests and in the sustainable development of rural Latin America [23-24, 31].
A literature review showed that many reports on the characterization of the chicle can be found over the past several decades [15,17,25-29,32-39]. Nevertheless, we are not aware that any work has been performed on the complete characterization of the chicle gum (Manilkara zapota) from the tropical rainforest of the Great Peten in the Yucatan peninsula (Mexico) nor that this renewable resource has been used in metathesis transformations.
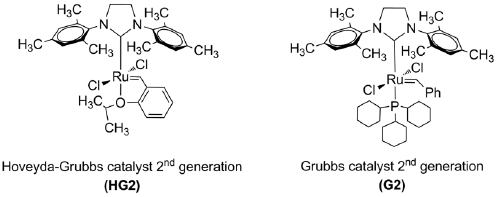
In this study, we performed for the first time the self-metathesis of the chicle gum and cross-metathesis of the chicle gum rubber, isolated from the gummy latex of sapodilla (Manilkara zapota) with orange oil, β-pinene and, methyl methacrylate using the Ru-alkylidenes (1,3-bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene) dichloro (o-isopropoxyphenyl methylene) ruthenium (Hoveyda-Grubbs catalyst second Generation, HG2) and (1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene) dichloro (phenylmethylene) (tricyclohexylphosphine) ruthenium (Grubbs catalyst second generation, G2). The chicle gum (Manilkara zapota) from the Great Peten tropical rainforest in the Yucatan peninsula (Mexico) and the obtained end-functionalized oligoisoprenes were characterized using the following techniques: (1) wide-angle X-ray scattering (WAXS), (2) thermogravimetric analysis (TGA), (3) differential scanning calorimetry (DSC), (4) nuclear magnetic resonance (NMR), (5) size exclusion chromatography (SEC), (6) matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) and (7) gas chromatography mass spectrometry (GC-MS).
Experimental
Reagents
Chicle gum base from the tropical rainforest of the Great Peten in Yucatan peninsula (southeastern Mexico neighboring Belize and Guatemala), tapped from the chicozapote (Manilkara zapota) was purchased from Consorcio Chiclero, SC de RL. Orange oil (d-limonene 96%, β-myrcene 4%) was purchased from 3QR Materias S.A. de C.V. and was used without purification. The (1,3-bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene) dichloro (o-isopropoxyphenylmethylene) ruthenium (Hoveyda-Grubbs catalyst second Generation, HG2), (1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene) dichloro (phenylmethylene) (tricyclohexylphosphine) ruthenium (Grubbs catalyst second generation, G2), β-pinene, methyl methacrylate (MMA), toluene anhydrous, tetrahydrofuran HPLC, acetone, ethyl acetate, toluene, methanol and other reagent grade solvents were purchased from Sigma-Aldrich and were used without further purification.
Characterization
Wide-angle X-ray scattering (WAXS) measurements were carried out in air at room temperature, using a Bruker D-8 Advance diffractometer with the Bragg-Brentano θ-θ geometry, CuKα radiation, a Ni 0.5% Cu-Kβ filter in the secondary beam, and a one-dimensional position-sensitive silicon strip detector (Bruker, Lynxeye). The diffraction intensity as a function of 2θ angle was measured between 2º and 30º, with a 2θ step of 0.02037º, for 0.4 s per point.
Thermogravimetric analysis (TGA) was performed on a TA Instrument (TGA Q5000 IR) with a heating rate 10 ºC/min, under nitrogen, in the range 25-500 ºC. The sample weight was about 5 mg.
Differential Scanning Calorimeter (DSC) was carried out on a TA Instrument (DSC Q2000). The sealed aluminum pan containing the sample was loaded into the DSC instrument with an identical empty pan used as the reference. The sample weight was 5 mg approximately and it was heated from -88 to 100 ºC at a heating rate of 10 ºC/min.
ATR-FTIR spectra were recorded at room temperature with 4 cm−1 resolution and scanned 32 times on a Nicolet 5700 spectrophotometer with a diamond tip as a dispersing agent.
1H and 13C NMR spectra were recorded on a Varian Inova Unit 300 (300 and 75 MHz, respectively) spectrometer. The samples were dissolved in CDCl3. Chemical shifts are reported in ppm downfield from tetramethylsilane (Me4Si = 0.00 ppm).
The number-average molecular weight (Mn), the weight-average molecular weight (Mw) and molecular weight distributions (Mw/Mn) were determined by size exclusion chromatography (SEC). The SEC analysis was performed at 35 ºC in THF (0.3 ml/min) using a Waters ALLIANCE 2695 Separation Module equipped with 2 columns: Waters Styragel HR 4E (Mw 5x102 to 1x105) and Styragel HR 5E (Mw 2x103 to 4x106) and a differential refractometer detector. Narrow molecular weight linear polystyrene (PS) standards (ranging from 3.70x102 to 4.290x106) were used to calibrate the SEC.
The matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrum was registered on a Bruker Microflex spectrometer equipped with a nitrogen laser (λ=337nm). The spectrograms were obtained in linear mode with an acceleration tension of 19kV. The irradiation targets were prepared from THF solutions using dithranol and AgNO3 (silver nitrate) as matrix and cationizing agent, respectively.
The GC-MS analysis was carried out using an Agilent Technologies 6890N gas chromatograph with capillary column HP5 (30 m x 0.25 mm, 0.25 μm film thickness). It was coupled to a JEOL JMS-GC mate II. The temperature was programmed at 40 ºC which was held for a minute and later increased to 310 ºC at a rate of 8 ºC/min for 6 min. The injector was set at 310 ºC. Helium was used as a carrier gas with a constant flow of 1 ml/min. The mass spectrometer was operated in electron impact (EI) scan mode at 70 eV. Component identification was based on the comparison of retention times and mass spectra with authentic standards. The concentration of pentacyclic triterpenes was estimated by measuring peak areas in ion chromatograms after calculating a correction factor between the peak area on the specific ion chromatogram and the peak area on the total ion current (TIC) chromatogram.
Purification of natural chicle gum
The chicle gum base or crude chicle was purified following a methodology previously described by Schlesinger and Leeper [26]. The inorganic constituents and carbohydrates were separated from the chicle polymers and resin by dissolving the chicle gum in toluene at 25º to 35 ºC, during 24 h with constant stirring. This mixture was then centrifuged to separate the transparent yellow-brown toluene solution, i.e., the refined chicle from the brown insoluble (residue). Thereafter, this refined chicle was treated with ethyl acetate and the mixture was refrigerated overnight to precipitate the rubber fraction (CR). This CR was filtered with suction on a Büchner funnel. Afterward, it was extracted in a Soxhlet apparatus with acetone for 24 h to remove waxy and resinous impurities. Subsequently, the trans-1,4-polyisoprene (TPI) extracted was dried in a vacuum oven until the weight was constant. This fraction was used for further analysis. The filtrate obtained by filtering off the CR was treated with acetone in order to precipitate the cis-1,4-polyisoprene (CPI). This polymer was recovered by decanting off the supernatant liquid and was extracted in a Soxhlet with acetone for 24 h. Later, it was dissolved in toluene, filtered through paper, and reprecipitated with acetone. Finally, it was dried under vacuum at room temperature. This CPI was used for further analysis.
The chicle resin was obtained by collecting and concentrating the ethyl acetate-acetone filtrate in a rotavapor. Then, it was dried in vacuo at room temperature. The resin had a yellow appearance and it crystallized spontaneously on standing.
Metathesis procedure
Self-metathesis of chicle (0.5 g, 1.2 mmol) and cross-metathesis of the rubber fraction of chicle (CR) (0.5 g, 7.4 mmol) with chicle resin, orange oil, β-pinene and, methyl methacrylate (MMA) as CTAs [C=C]/[CTA]=1:1, 2:1) were performed in toluene anhydrous at 10% (w/v), and in bulk conditions, i.e., the solvent was added only with the catalyst. Reactions were warmed to 80 °C in a preheated, thermostatted oil bath under nitrogen and magnetic stirring for 24 h. The catalyst Hoveyda-Grubbs second generation (HG2) or Grubbs second generation (G2) was added to the reaction vessel in molar ratios [C=C]/catalyst= 100/1, 200/1, 500/1 and 1000/1. The metathesis reaction was quenched by adding ethyl vinyl ether, the products were stabilized by adding N-phenyl-1-naphthylamine to the solution and then were precipitated using an excess of methanol. The products were dried under vacuum at room temperature and characterized by FT-IR, 1H and 13C NMR, SEC, and MALDI-TOF.
Results and Discussion
Characterization of the chicle gum (Manilkara zapota) from Yucatan (Mexico)
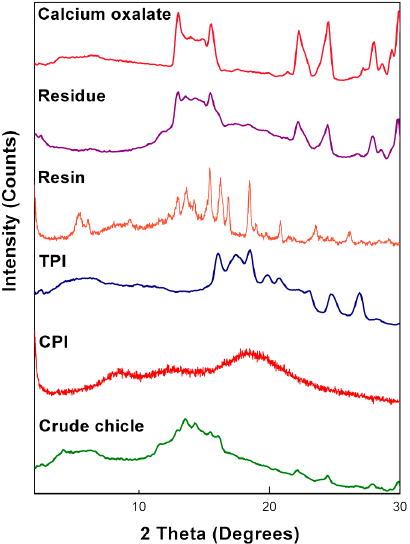
To further develop this renewable resource in the field of green chemistry, we needed to determine the structural information in detail. Therefore, the chicle gum was separated into three fractions: the polyisoprenes (TPI and CPI), the resin and the residue. This successful fractionation confirmed that these constituents are not chemically combined but constitute a physical mixture. Each fraction was characterized by wide-angle X-ray scattering. The WAXS diffractograms are shown in Fig. 1. The CPI shows a broad halo at 2= 20º which is the signature of the amorphous nature of the sample, whereas TPI is partially crystalline. The interplanar spacing has been calculated using the Bragg’s law from which two crystalline phases, namely the α and β polymorphic forms were identified [33,40]. The α-form has a monoclinic unit cell with two chains, each containing two repeat units (d= 7.82, 5.52, 5.09, 4.48, 4.28, 3.99, 3.60, 3.31, 2.95, 2.72 Å). The β-form has an orthorhombic unit cell with four chains, each containing one repeat unit (d= 4.80, 3.86, 2.95 Å) [41]. Respect to the resin it presents a crystalline pattern. The peaks of the residue corresponding to the same 2 positions of the pure calcium oxalate confirm the presence of calcium oxalate monohydrate as the constituent of the inorganic fraction as Stillwell had reported [33].
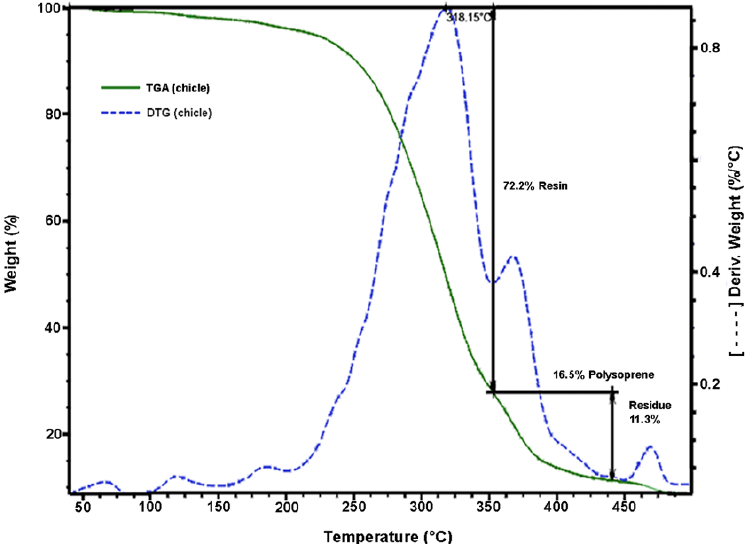
The chicle gum was subjected to thermogravimetric analysis. Fig. 2 shows the TGA/DTG thermograms of the chicle gum. It is observed that the mass loss occurs in three steps. The first step occurs in the range 50-350 °C with 72.2% of mass loss and refers to the resin. The second step is observed between 350-438 °C with 16.5% of mass loss, which is attributed to the degradation of cis-1,4- and trans-1,4-polyisoprenes. The 11.3% of residual mass at the end of the thermal degradation process (500 °C) is related to inorganic constituents. This result is in accordance with the chicle gum fractionation procedure.
The rubber fraction of chicle and the isolated TPI and CPI polymers were analyzed through differential scanning calorimetry (DSC), infrared spectroscopy (FT-IR), 1H and 13C nuclear magnetic resonance (NMR) and size exclusion chromatography (SEC). Figures 3a and 3b show the DSC thermograms for TPI and CPI, respectively. The two polymers are very different in physical appearance at room temperature, the trans isomer (TPI) is crystalline and tough, while the cis (CPI) is amorphous and elastic. The Tg for a semi-crystalline polymer such as TPI is broader (and the magnitude of the (Cp is markedly reduced) than the glass transition observed for the amorphous sample CPI (Tg= -61.92 ºC) [42]. Fig. 3a indicates the presence of both α and β forms, as represented by two distinguished peaks (95.68 J/g) at Tendo= 64.92 ºC and Tendo= 60.67 ºC, respectively, with predominantly α-form [26,35,40]. Whereas in Fig. 3b the peak at Tendo= 58.29 ºC with a significant difference in the enthalpy value 10.57 J/g could be attributed to TPI since a pure cis polymer was not obtained by the fractionation, as can be noticed in the 1H NMR spectrum (Fig. 4c).
Additionally, the infrared analysis of TPI and CPI presented characteristic bands of 1,4-units around 836 (CH out-of-plane wagging (-CCH3=CH)), 980 (C-CH3 stretching), 1446 (CH2 deformation), 1664 (C=C stretching), 2851 (CH2 symmetrical stretching), 2912 (CH2 asymmetrical stretching) and 2960 (CH3 asymmetrical stretching) cm-1. Some other specific bands of 1,4-cis units appeared at 572, 742, 1130, and 1315 cm-1. For 1,4-trans units, peaks were observed at 600, 801, 1152 and 1325 cm-1. FT-IR also allowed the identification of the bands related to the α (750, 1030, 1205, 1340, 2872, and 2879 cm-1) and β (750, 997, 1212, 1348, 2906, and 2914 cm-1) forms of TPI [26,34,38,43].
NMR has been found to be more accurate than the methods based on infrared analysis when determining the cis/trans ratio in polyisoprene mixtures. Accordingly, the relative areas of the methyl proton signals (δ=1.67 and 1.60 ppm for CPI and TPI, respectively) of the isolated chicle rubber were measured and the cis/trans ratio was estimated to be 25/75 [44]. Fig. 4 shows the 1H NMR spectra of chicle gum, TPI and CPI fractions with their corresponding assignments. The 13C NMR of the TPI presents the chemical shifts in CDCl3 δ (ppm): 134.93 (C-2), 124.23 (C-3), 39.75 (C-1), 26.73 (C-4), 16.03 (C-5). For CPI δ (ppm): 135.18 (C-2), 125.0 (C-3), 32.19 (C-1), 26.38 (C-4), 23.42 (C-5). The absence of splitting in C1 and C2 signals from the TPI and CPI spectra indicates that chicle contains both pure cis and trans-PI and constitute merely a physical mixture. These results are in agreement with previous findings [26,37,39].
Fig. 5a presents the SEC chromatograms for the CPI, TPI and refined chicle, i.e., the polymer and resin fractions isolated from the chicle gum. The CPI molecular weight was estimated to be Mn= 1.3x105 g/mol with a polydispersity of Mw/Mn= 2.9 and exhibited a bimodal distribution. It can be noticed that TPI showed a monomodal distribution with Mn= 1.5x104 g/mol and Mw/Mn= 1.6, similar to the molecular weight of the low weight peak of CPI. The molecular weight distribution of the refined chicle is referred to the presence of CPI, TPI and the resin of low molecular weight. Tanaka et al. [39] reported the values of Mn= 1.4x105 g/mol and Mw/Mn= 1.3 for CPI, and Mn=7.9x103 g/mol and Mw/Mn= 1.2 for TPI, using low-angle laser light scattering photometer (LALLS) equipped with a refractive index (RI) detector. Whereas, Hager et al. [27] using an RI detector obtained Mn= 1.5x105 and Mn≈ 104 g/mol for CPI and TPI, respectively. Our results for TPI and CPI could be attributed to the fact that we used the RI detector thus the obtained values were related to PS standards.
One of the key advantages of using MALDI-TOF MS for polymer analysis is that the absolute molecular weights of oligomers can be determined as opposed to obtaining relative molecular weights through chromatographic techniques [45]. Consequently, the chicle gum was analyzed by MALDI-TOF and the spectrum is shown in the Fig. 5b. The sample peaks identified the mass corresponding to the polyisoprene molecules ionized with silver (Ag+). The difference between the peaks in the spectrum is equal to the mass of the repeating unit (Misoprene=68). The molecular masses were calculated using the equations reported elsewhere [46], and the values were Mn=7,990, Mw= 8,373 and Mw/Mn= 1.05. As we were expecting, we obtained a low molecular weight distribution since TPI is the main component of the polymer mixture. In this respect, it has been reported for synthetic trans-1,4-PI that the true Mn is actually half the SEC values determined by PS standards [47]. Therefore, our SEC corrected values for the TPI fraction could be in accordance with the Mn distribution previously reported [27,39] and with the MALDI-TOF outcome that we obtained.
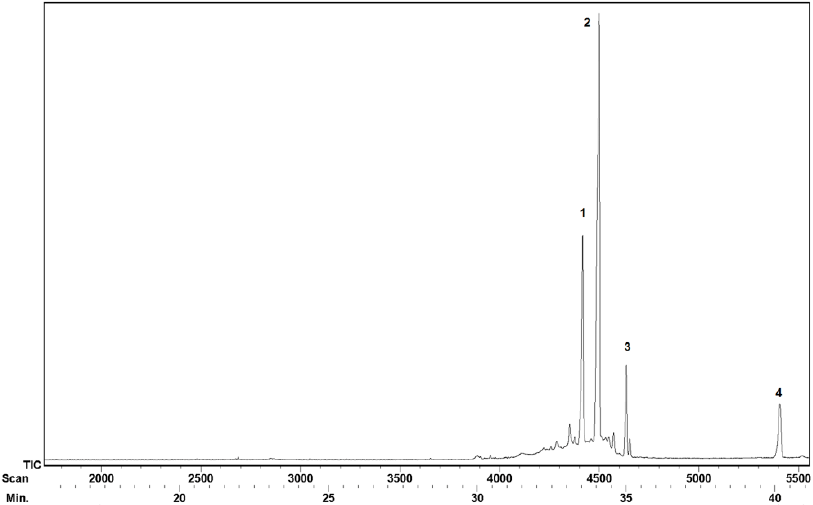
Finally, the chicle resin fraction was analyzed through GC-MS and FT-IR. The GC-MS analysis indicated that four pentacyclic triterpenes eluted in the 33-41 min tR range under our GC conditions (Fig. 6).
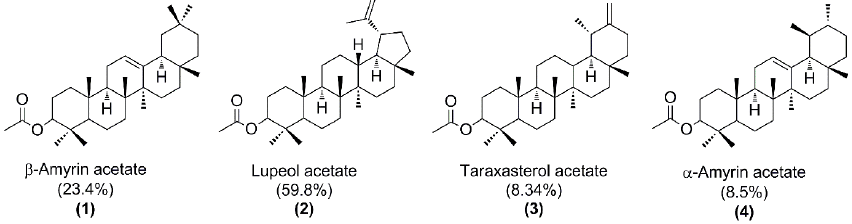
Fig. 7 shows the four pentacyclic triterpenes present in the chicle resin. The identification of the GC peaks was made by comparison of the tR and MS with authentic standards. Our findings are consistent with the previously reported for chicle from Campeche and Guatemala [17,28-29].
The FT-IR of the chicle resin presents the characteristic bands: 880 (-C=CH2), 980 (C-CH3 stretching), 1024 (CH3 rocking), 1242 (C-O-C asymmetrical stretching), 1362 (CH3 asymmetrical deformation), 1454 (CH2 deformation), 1643 (C=C symmetrical stretching), 1729 (C=O stretching), 2852 (CH2 symmetrical stretching), 2927 (CH2 asymmetrical stretching), 3063 (-C=CH2) cm-1.
Metathesis transformations of the chicle gum and chicle rubber fraction (CR)
Scheme 1 depicts the self-metathesis of chicle and the cross-metathesis of CR with orange oil (d-limonene 96%), β-pinene and methyl methacrylate (MMA) as CTAs, catalyzed by the Ru-alkylidenes HG2 and G2 (Fig. 8).
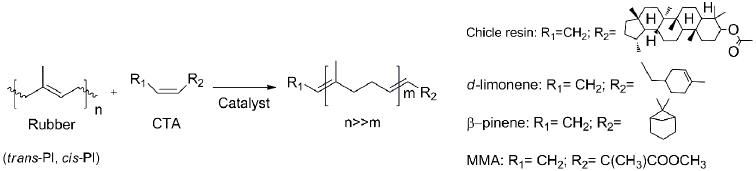
Scheme 1 Self-metathesis of chicle and cross-metathesis of CR with orange oil, β-pinene and MMA (CTAs) catalyzed by HG2 and G2.
Table 1 summarizes the results of the self-metathesis of chicle (entries 1-2) and cross-metathesis of CR with chicle resin (59.8% lupeol acetate), orange oil (d-limonene 96%), β-pinene and, MMA as CTAs catalyzed by HG2 and G2 (entries 3-9). Reactions were performed in toluene anhydrous at 10% (w/v) and in bulk conditions, at T= 80 ºC for t=24 h. In order to prove that the resin was participating as CTA, it was carried out the cross-metathesis of the isolated CR with the chicle resin (entry 3). The catalyst loads (200/1, 1000/1 and, 100/1) permitted the synthesis of the desired ester-terminated oligomers in high yields (79-89%) and selective manner, as it was confirmed by 1H NMR (Fig. 9). The second-generation catalysts HG2 and G2 (500/1) exhibited high activity in the cross-metathesis reactions of CR with orange oil, β-pinene and MMA, and afforded the monoterpene and methyl ester end-functionalized oligoisoprenes with high yield (73-93%) and selectivity. The molar masses Mn ranged from 612 to 4,839 g/mol and Mw/Mn = 1.3-2.3 as it was confirmed by FT-IR, 1H and 13C NMR, SEC and MALDI-TOF (Figures 10- (11). The MMA was found a more suitable cross-metathesis partner since it synthesized the semi-telechelic oligomers with the lowest molecular weight compared to the pentacyclic triterpene and monoterpene end-capped products.
Table 1 Self-metathesis of chicle and cross-metathesis of CR with chicle resin, orange oil, β-pinene and MMA using HG2 and G2 as catalysts (T= 80 ºC, t=24 h).
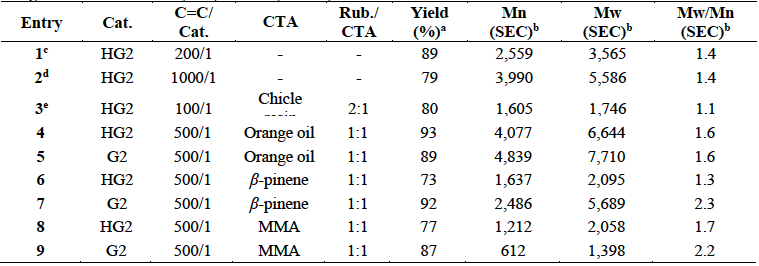
CR: Chicle rubber fraction: TPI (Mn=1.5x104 g/mol, Mw/Mn=1.6), CPI (Mn= 1.3x105 g/mol, Mw/Mn=2.9).
HG2: Second generation Hoveyda-Grubbs catalyst.
G2: Second generation Grubbs catalyst.
a: Weight percent yield of oligomers.
b: Determined by SEC with THF as the eluent. Values reported relative to PS standards.
c: Chicle gum (72.2% resin, 12.4% trans-PI and 4.1% cis-PI) was dissolved in toluene at concentration 10% (w/v).
d: Refined chicle (18.6% polyisoprenes and 81.4% resin) was dissolved in toluene at 10% (w/v).
e: CR was dissolved in toluene at 10% (w/v).
Entries 4-9: Reactions were performed in bulk conditions.
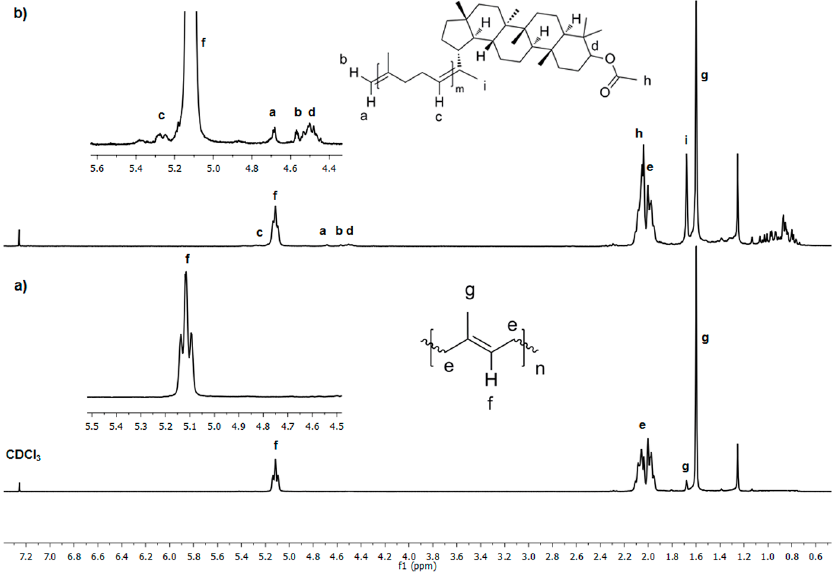
Fig. 9 1 H NMR (300 MHz, CDCl3) spectra of a) initial CR and b) after 24 h of cross-metathesis of CR with chicle resin (Table 1, entry 3).
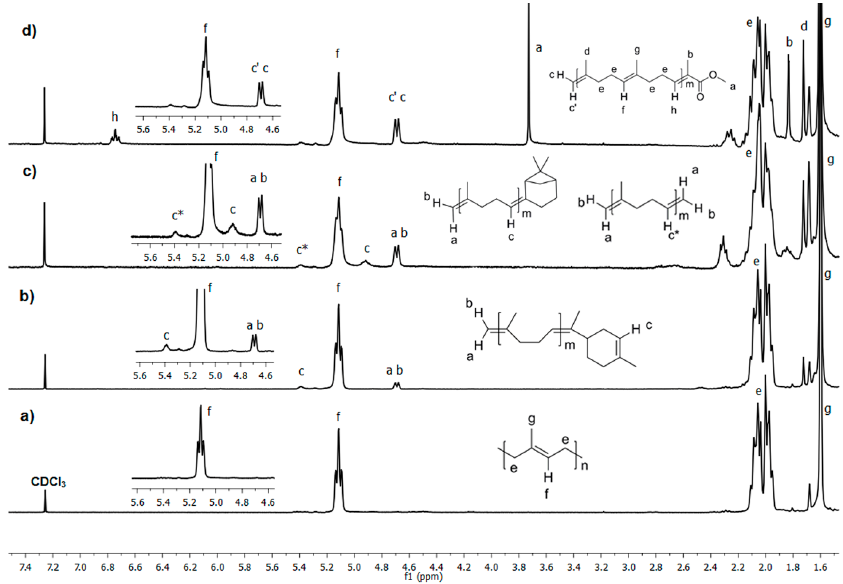
Fig. 10 1H NMR (300 MHz, CDCl3) spectra of a) initial CR and the cross-metathesis products with b) orange oil, c) β-pinene and d) MMA as CTAs catalyzed by HG2 (Table 1, entries 4, 6 and 8).
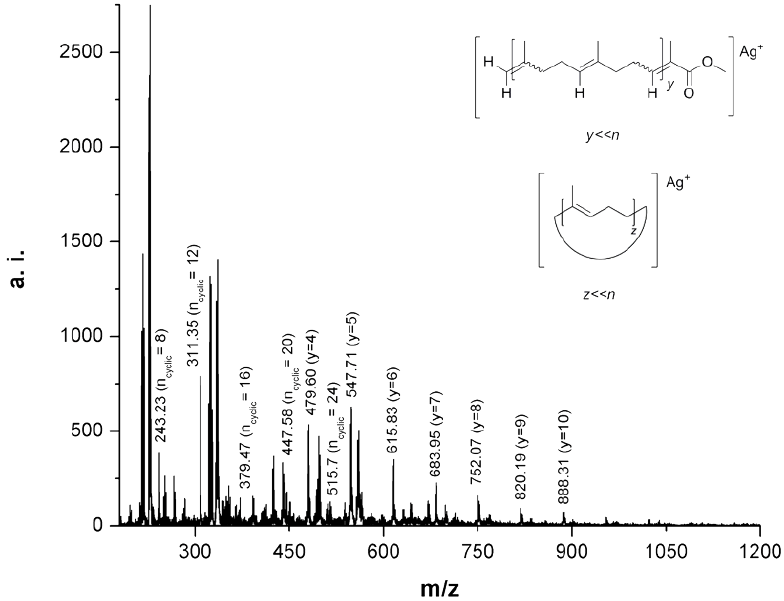
Fig. 11 MALDI-TOF MS of the oligoisoprene products after cross-metathesis of CR with MMA (Table 1, entry 8).
Fig. 9 shows the 1H NMR spectra of a) initial CR and b) after 24 h of cross-metathesis of CR with chicle resin catalyzed by HG2 (Table 1, entry 3). The initial CR shows chemical shifts at δ= 5.12 (CH=C), 2.06, 2.00 (CH2), 1.60 (CH3) and a small peak at 1.67 ppm (CH3) due to residual CPI. The peak at δ= 1.25 ppm is referred to residual wax from the resin. Fig. 9b presents the chemical shifts of the pentacyclic triterpene end-capped oligomers. New peaks due to olefin (δ=5.25 ppm) and vinyl protons appeared at δ= 4.69 and 4.68 ppm. The methine proton on 3-position binding acetyl group is observed at δ 4.48 ppm. The acetyl group appeared at 2.05 ppm and the presence of methyl signals at δ ppm: 1.69 (H-30), 1.03 (H-25), 0.94 (H-28), 0.87 (H-23), 0.85 (H-24), 0.84 (H-26) and, 0.80 (H-27). The peaks below 2 ppm for aliphatic (CH3, CH2, and CH) were assigned to the pentacyclic triterpene skeleton. The spectra of the products from self-metathesis of chicle and cross-metathesis of CR with chicle resin were identical, thus confirming the selective formation of the ester end-functionalized oligoisoprenes.
Fig. 10 presents the 1H NMR spectra of CR before and after 24 h of degradation with orange oil, β-pinene and, MMA as CTAs catalyzed by HG2 (Table 1, entries 4, 6 and 8). Fig. 10a shows chemical shifts due to TPI and CPI polymers at δ= 5.12 (CH=C), 2.06, 2.00 (CH2), 1.67, 1.60 ppm (CH3). When orange oil (d-limonene 96%) was used as the cross-metathesis partner, new signals of C=CH protons of the cyclic monoterpene appeared at 5.39 ppm. It also showed the signals of C=CH2 protons at δ= 4.68 and 4.70 ppm. The signals of the aliphatic protons of d-limonene are observed at 1.4-1.1 ppm. For β-pinene, there were observed new peaks in the sp2 zone at δ= 5.39, 4.92, 4.70 and 4.68 ppm. The protons at δ= 5.39 (c*), 4.70 (a) and 4.68 (b) ppm were assigned to the bis-vinyl oligomer product; while the signals arising at δ= 4.92 (c), 4.70 (a) and 4.68 (b) ppm correspond to the cyclic monoterpene and vinyl terminated product. The peaks in the 1.2-0.9 ppm region were attributed to aliphatic protons of the terpene-terminated oligomer. Finally, when using MMA, the spectrum shows new peaks at δ=4.70-4.68 (C=CH2) and 3.73 ppm (CH3-OCO). These observations confirmed the formation of monoterpene and methyl ester terminated isoprene oligomers.
The MALDI-TOF MS of the oligomers isolated after precipitation of the cross-metathesis reaction of CR with MMA catalyzed by HG2 is shown in Fig. 11 (Table 1, entry 8). It can be noted that the difference between the main peaks in the spectrum is equal to the mass of isoprene (M= 68) as in the case of the chicle gum (Fig. 5b). Two types of products ionized with silver (Ag+) were identified from the spectrum, the methyl ester end-functionalized and the macrocycles. The presence of macrocycles could be explained by the intramolecular reaction of the polyisoprene chains [48]. The experimental values are in good agreement with the theoretical masses calculated with the following equation: M=M(end group) + M(Ag) + mM(repeating unit). The molecular mass distribution was estimated to be Mn= 438, Mw= 831 with a polydispersity of 1.9. While using G2 a higher dispersity was obtained Mn= 429, Mw= 1,715 and Mw/Mn= 4.0. Again, these MALDI-TOF outcomes are lower than those determined by SEC related to PS (Table 1, entries 8 and 9).
Conclusions
We studied for the first time, the self-metathesis of the chicle gum and cross-metathesis of the chicle gum rubber isolated from the gummy latex of sapodilla (Manilkara zapota) with orange oil, β-pinene and methyl methacrylate using the Ru-alkylidenes Hoveyda-Grubbs and Grubbs second generation catalysts. The chicle rubber can undergo efficient cleavage reactions with the resin (59.8% lupeol acetate) yielding ester-terminated oligoisoprenes, demonstrating that the pentacyclic triterpenes participate as cross-metathesis partners. The second-generation catalysts HG2 and G2 exhibited high activity toward the self-metathesis of chicle and cross-metathesis of chicle rubber to give terpene- and ester- end-capped oligomers with good yields (73-93%) and selectivity. Methyl methacrylate was found a more suitable cross-metathesis co-reactant since it afforded products with the lowest molecular weight compared to those obtained when orange oil and β-pinene were used as a CTA. We believe that chicle has great potential as a renewable feedstock for the synthesis of telechelic oligomers and polymers.











 nueva página del texto (beta)
nueva página del texto (beta)