Introduction
Sapium is a cosmopolitan genus of the Euphorbiaceae plant family which includes 23 accepted names of species, consolidated from 254 former names of synonyms. Several species of this genus have been used in traditional medicine in several parts of the world for treating wounds, snake bites, and skin-related diseases, among other ailments [1,2]. From Sapium species collected in Asia, phenolic compounds were identified as constituents of S. insigne[3,4, S. sebiferum[5,6; phorbol esters were identified as secondary metabolites of S. indicum[7,8] and S. insigne[3,9]; and the alkaloid bukittinggine and triterpenes were identified as constituents of S. baccatum10-12. Among species collected in the American continent, terpenoids have been isolated from S. rigidifolium[13] and S. haematospermum[14], and coumarins from S. sebiferum[15].
S. macrocarpum is a tree native mainly to the southern part of Mexico and Central America. It is used in Mayan traditional medicine to cure skin infections, and particularly, for treating warts [16]. It has not been chemically analyzed previously, but some phytotoxic compounds have been isolated from an endophytic fungus of this species [17]. Here we report the chemical constituents isolated from an extract of the aerial parts of S. macrocarpum, which displayed cytotoxicity in selected tumor cell lines. We identified tonantzitlolone A (7), an uncommon diterpene with a flexibilane skeleton, together with a series of known compounds, and determined their cytotoxicities.
Results and Discussion
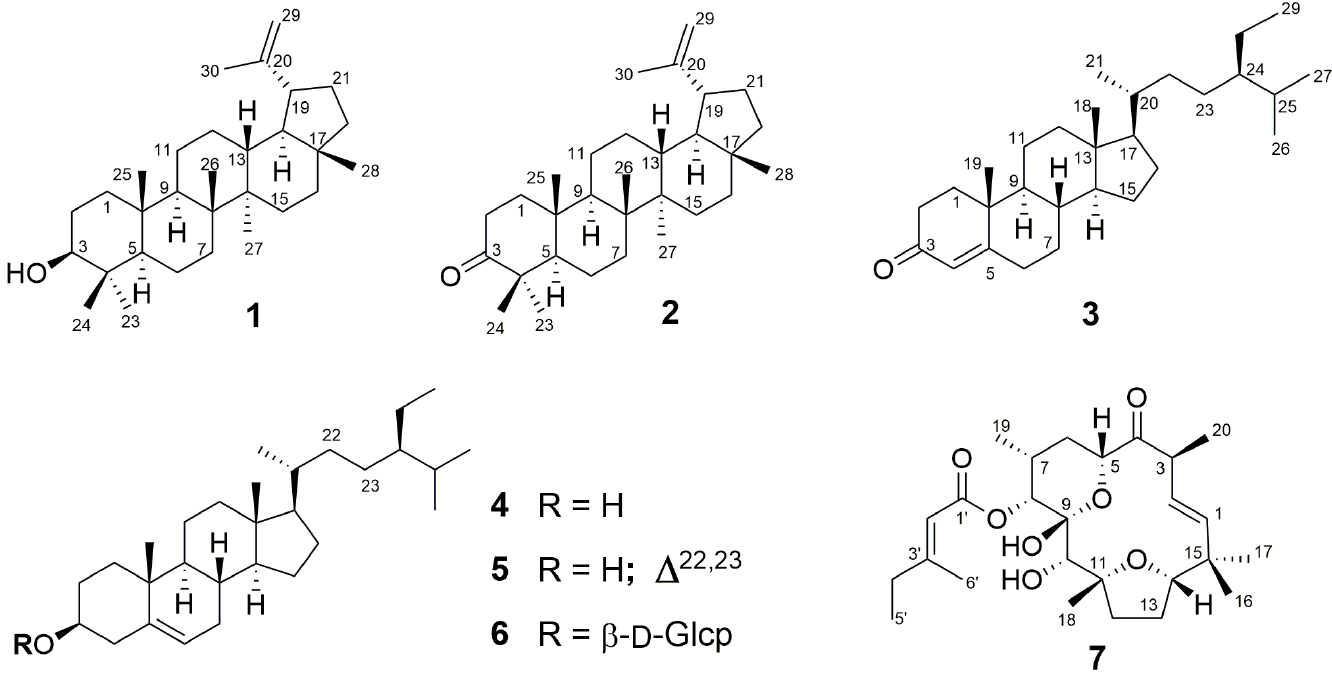
The 13C NMR spectrum of compound 1 showed 30 signals which were defined by DEPT experiments. The results showed seven methyl groups, eleven methylenes (one vinylic methylene), six methines (one oxymethine), and six quaternary carbons. The 1H NMR spectrum displayed six singlets for methyl groups between δH 1.03 and δH 0.76, and one methyl group at δH 1.68; the latter signal belonged to an isopropenyl fragment, consistent with the presence of the vinylic methylene mentioned above. These NMR data were identical to those reported for lupeol (1) [18], and a direct comparison with an authentic sample confirmed its structure. The less polar fractions of the chromatogram provided a white solid. Its 13C NMR spectrum also displayed 30 signals that included a carbonyl group of a ketone (δC 218.40) and signals with chemical shifts similar to those for 1, but did not show the oxymethine observed for 1 (δC 79.02). The EIMS showed a molecular ion at m/z 424 in agreement with the molecular formula C30H48O. The 13C NMR data and its physical constants were identical to those reported for lupenone (2), confirming its structure [19]. Four structurally related compounds were isolated, whose 1H and 13C NMR data indicated the presence of four tertiary methyls, three secondary methyls and one primary methyl, characteristic for phytosterols. Sitostenone (3) [20,21], β-sitosterol (4), stigmasterol (5)[22] and β-sitosteryl β-D-glucopyranoside (6) [23] were characterized. The identities of these compounds were confirmed by comparing their 1H and 13C NMR data and their physical constants with those published in the literature, and with authentic samples available in our laboratory.
The molecular formula of C26H40O7 for metabolite 7, was established from the analysis of its NMR and EIMS data. The 13C NMR spectra (Table 1) exhibited 26 carbon signals, which comprised seven methyl groups (three tertiary, two secondary, one primary and one vinylic), four methylenes, nine methines (four bonded to oxygen, three olefinic, and two aliphatic), and six non-protonated carbons (two carbonyls, one olefinic, one aliphatic, one bonded to oxygen, and one bonded to two oxygens). The presence of a methyl senecioate fragment (C6H9O2) was established by the 13C and 1H NMR signals assigned (by HMBC analysis) to an ester carbonyl (δC 166.58) bonded to the olefinic methine (δC 113.86, δH 5.70) of a trisubstituted double bond (δC 162.97), having an ethyl (δC 33.88, δH 2.17; δC 11.83, δH 1.07) and a methyl group (δC 18.99, δH 2.16) as the other substituents. Therefore, this compound was a diterpene with five degrees of unsaturation bonded to a methyl senecioate fragment. The presence of a β,γ-unsaturated-α-methyl ketone fragment was deduced from the 13C and 1H NMR signals of a ketone (δC 211.30) bonded to a methine (δH 3.34, according to HMBC correlation), which was further linked to a methyl group (δC 16.00, δH 1.12, HMBC and COSY correlations) and to a trans disubstituted double bond (δC 126.81, δH 5.24; δC 140.07, δH 5.86). In addition, the remaining 1H and 13C NMR signals, particularly those for two aliphatic methylenes, three oxymethines, and one hemiketal carbon, were consistent with the presence of a δ-lactol and a tetrahydrofuran in the diterpene skeleton. The NMR data were almost identical to those reported for tonantzitlolone (7, named so after the Aztec goddess Tonantzin, by Dr. X. A. Domínguez), a metabolite previously isolated from Stillingia sanguinolenta, an endemic plant used traditionally in northern Mexico [24]. However, a number of inconsistencies in the reported 1H NMR assignments were detected, particularly those for the H-12α and H-12β, and the H-13α and H-13β [25-27]. The correct assignments are shown in Table 1. These assignments were based on the analysis of the coupling constants between the hydrogens of the tetrahydrofuran ring, which indicated that the observed J 12α,13β = 0 (not informed in references 24-27) was in agreement with the E2 arrangement of the conformational itinerary of an oxolane ring [28]. Consequently, some reported NOESY correlations should also be corrected according to the new assignments. For instance, the NOESY crosspeak between H-14 (δH 3.77) and the signal at δH 2.45 informed by Lima [26], was not detected, instead, a correlation between H-14 and H-13β (δH 1.77) was clearly observed. Some minor inconsistencies were due to the use of different numbering systems for the macrocycle, in addition to the fact that one of the first structures published was later corrected [29]. Selected relevant NOESY correlations are illustrated in the X-ray structure depicted in Figure 1(a), since the conformation determined by X-ray analysis (vide infra) was in agreement with the H-H dihedral angles deduced from the J H-H 1H NMR coupling constants. Some fragments of this compound were previously synthesized [30], the total synthesis has also been completed [24,31], and its absolute configuration has been established [32], as depicted in formula 7.

Figure 1 (a) Selected NOESY correlations of tonantzitlolone A (7) using the drawing derived of the X-ray analysis. (b) X-ray crystal structure of tonantzitlolone A (7) indicating the intramolecular hydrogen bonds (by broken lines).
An X-ray analysis of compound 7 confirmed the molecular connectivity and the absolute configuration. In addition, we could identify two intramolecular hydrogen bonds: one between OH-9 and the tetrahydrofuranic oxygen, and the second between OH-10 and the pyranyl oxygen (Figure 1 (b) ). On the basis of these findings, the structure of 7 could be considered to have some rigidity, which further supported the observed 1H-1H NMR coupling constants, consistent with the conformation of the metabolite in the solid state. The absolute configuration for this compound was the same as that obtained for a compound isolated from Stillingia lineata (Euphorbiaceae) in the search of antichikunguya agents; and thus, compound 7 was renamed by Grondin et al. as tonantzitlolone A [27], since Kirshning et al. previously characterized an structural variant with difference in the ester side chain, named tonantzitlolone B [25].
The inhibitory activities of the extract of Sapium macrocarpum and of compounds 1-3, 6, and 7 were tested against U251, PC-3, K562, HCT-15, MCF-7, and SKLU-1 human tumor cell lines using the protein-binding dye sulforhodamine B (SRB) microculture assay to measure cell growth [33]. The results revealed that the extract (at 50 μg / mL) and the assayed compounds (at 50 μM) displayed differential activity (Table 2). Lupeol (1) exhibited clear activity against PC-3 cells, and all the natural compounds (1-3, 6 and 7) were active against the leukemia cell line (K562). β-Sitosteryl β-D-glucopyranoside (6) displayed the highest activity in the K562 cell line (77% of inhibition), consistent with results with other leukemia cells (CEM/ADR5000) [34]. However, evaluations of these pure compounds against other tumor cell lines indicated inhibitory activities below 40%. Sitostenone (3) was previously reported to possess anti-proliferation and anti-tyrosinase activities in melanoma cells [35]. The activities displayed by tonantzi tlolone A (7) were similar to those reported previously for tonantzitlolones B-F [36]. Recently, tonantzitlolone A (7) was identified as a potent anti-tumor agent in renal cancer cells as a PKC activator [37], and as a cytostatic compound on tumor cells, inducing monoastral half-spindle formation [38].
Conclusion
In our search for bioactive natural products, we found that the extract of S. macrocarpum (collected in the State of Chiapas, Mexico), a plant used in Mayan traditional medicine for the treatment of warts [16], displayed inhibitory activity toward some cancer cell lines. This species had not been previously analyzed, and in the present chemical investigation we characterized compounds 1-7. The 1H NMR analysis of the secondary metabolite 7 allowed the reassignments of the tetrahydrofuran hydrogens. Tonantzitlolone A (7) was previously isolated from a Mexican population of Stillingia sanguinolenta (collected in the State of Nuevo León) [25], and later from Sebastiania ma crocarpa (from the Brazilian State of Ceará) [26], and from Stillingia lineata (from La Reunion Island, Indian Ocean, France) [27. The cytotoxic evaluations of the chemical constituents showed activity and selectivity for some cell lines, although the extract displayed in some cases higher activity, may be due to synergistic interactions. The best cytotoxicities were found with tonantzitlolone A (7) and sitostenone (3), which showed clear activity against a leukemia cell line. The cytotoxicity results for tonantzitlolone A (7) were comparable to those found for tonantzitlonones B-F in a previous study [36]. It is interesting to note that flexibilane diterpenes have been characterized from Stillingia, Sebastiania and Sapium species; all these genera belong to the Hippomaninae subtribe, tribe Hippomaneae, subfamily Euphorbioideae of the family Euphorbiaceae [39].
Experimental
General Experimental Procedures
Analytical TLC was carried out on precoated silica gel 60 F254 sheets (Merck), and revealed with cerium ammonium sulfate (1%) in sulfuric acid 2N. For preparative chromatography (PLC) were used glass plates (20 x 20 cm) on precoated silicagel 60 F254, 2mm (Merck). Column chromatography (CC) was performed using silica gel (230-400 mesh). All solvents were dried and distilled before use. Melting points were determined on a Fisher Johns apparatus (Cole Parmer) and are uncorrected. 1H (400 MHz), 13C (100.0 MHz), and 2D NMR spectra (in CDCl3) were obtained on Bruker Avance III NMR 400 spectrometer. Chemical shifts were expressed in parts per million ( δ ) relative to TMS as internal standard. EIMS were taken in a JEOL JMS-AX 505 HA. Analysis by X-ray diffraction was performed using a Bruker D8 Venture automatic diffractometer with a CCD area detector at 173(2) K using Helios multilayer mirror Cu Kα radiation (λ = 1.54178Å).
Plant material, extraction and isolation
The aerial parts of S. macrocarpum Müll. Arg. was collected in Arriaga district, in the state of Chiapas, Mexico, in 2007. Prof. Esteban M. Martínez identified the plant material and a voucher specimen (39273 Esteban M. Martínez) was deposited in the collection of the National Herbarium (MEXU), Instituto de Bio logía, UNAM.
Aerial parts of S. macrocarpum (leaves and branches, 1.1 kg) were dried, powdered and extracted three times at room temperature with a mixture of CH2Cl2-methanol (1:1), and a residue (41 g) was obtained after evaporating the solvent under reduced pressure. Part of this residue (38 g) was adsorbed in a 1:1 mixture of celite and silica gel (30 g) and chromatographed over a silica gel column eluted with hexane-EtOAc mixtures of increasing polarity, yielding 130 eluates which were joined in eight main fractions (A-H), according TLC analyses. Fraction D (124 mg) was subjected to column chromatography over silica gel with hexane-EtOAc gradient system, to give a fraction that was further purified by preparative TLC eluted with a mixture of hexane-EtOAc (85:15), yielding lupenone (2, 15 mg). (Rf 0.69, hexane-EtOAc, 85:15). EIMS m/z (% rel): 424 (M+, 6), 355 (4), 313 (4), 245 (3), 232 (4), 205 (100), 189 (30), 109 (60), 95 (58), 69 (45), 67 (47). 1H NMR (CDCl3, 400 MHz): δ 4.63 (1H, d, J = 2.4 Hz, H-29b), 4.50 (1H, dd, J = 2.6, 1.4 Hz, H-29a), 2.35 (1H, m, H-19), 1.62 (3H, s, H-30), 1.50 (1H, s, H-2b), 1.37 (1H, s , H-11), 1.00 (3H, s, CH3-26), 0.96 (6H, s, CH3-23, CH3-27), 0.81 (3H, s, CH3-25), 0.79 (3H, s, CH3-24), 0.73 (3H, s, CH3-28). 13C NMR (CDCl3, 100 MHz): δ 218.4 (C-3), 151.1 (C-20), 109.6 (C-29), 55.1 (C-5), 49.9 (C-9), 48.4 (C-18), 48.1 (C-19), 47.5 (C-4), 43.2 (C-17), 43.1 (C-14), 40.9 (C-8), 40.1 (C-22), 39.8 (C-1), 38.3 (C-13), 37.0 (C-10), 35.7 (C-16), 34.3 (C-2), 33.7 (C-7), 29.9 (C-21), 27.6 (C- 15), 26.8 (C-23), 25.3 (C-12), 21.6 (C-11), 21.2 (C-24), 19.8 (C-6), 19.5 (C-30), 18.2 (C-28), 16.1 (C-25), 15.9 (C-26), 14.6 (C-27).
Fraction E (170 mg) was subjected to silica gel column chromatography using a hexane-EtOAc gradient to afford 92 fractions. Subfractions E27-E30 (20 mg) were combined and subjected to preparative TLC to yield tonantzitlolone A (7, 8 mg) as colorless crystals (from acetone). Rf 0.59 (hexane-EtOAc, 85:15), mp 172-173 oC. EIMS m/z (% rel): 464 (M+, 25), 446 (29), 364 (12), 350 (10), 180 (25), 126 (25), 97 (100), 69 (65). 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz), see Table 1. Subfractions E38-E47 were combined and the residue (29 mg) was subjected to preparative TLC to yield sitostenone (3, 9 mg). Rf 0.42, hexane:EtOAc 85:15. 1H NMR (CDCl3, 400 MHz): δ 5.72 (1H, s, H-4), 1.18 (3H, s, H - 19), 0.92 (3H, d, J = 8 Hz, H-21), 0.85 (3H, s, H-29), 0.83 (3H, d, J = 4 Hz, H-26), 0.81 (3H, s, H-27), 0.71 (3H, s, H-18). 13C NMR (CDCl3, 100 MHz): δ 199.91 (C-3), 171.9 (C-5), 123.9 (C-4), 56.7 (C-1), 56.2 (C-14), 56.0 (C-17), 54.0 (C-9), 45.97 (C-24), 42.5 (C-13), 39.8 (C-12), 38.8 (C-10), 36.3 (C-20), 35.8 (C-8), 34.1 (C-22), 34.0 (C-7), 33.1 (C-6), 32.2 (C-2), 29.3 (C-23), 28.4 (C-16), 26.2 (C-25), 24.3 (C-15), 23.21 (C-28), 21.2 (C-11), 19.97 (C-27), 19.2 (C-19), 18.9 (C-21), 17.54 (C-26), 12.1 (C-18, C-29).
Fraction F (440 mg) was rechromatographed over silica gel using hexane-EtOAc elution system to obtain 35 eluates. Subfractions F12-F18 (64 mg) were combined and rechromatographed over silica gel eluting with hexane-EtOAc gradient system, and some fractions were joined and further purified by preparative TLC to yield lupeol (1, 19 mg). Rf 0.40 (hexane-EtOAc, 85:15). From subfractions F24-F29 was obtained a solid that was characterized by 1H NMR as a 4.3:1 mixture of β-sitosterol (4) and stigmasterol (5) (72 mg), Rf 0.26, hexane-EtOAc, 85:15. Sequential recrystallizations from acetone-di-isopropyl ether-hexane allowed the purification of the compounds.
From fraction H was obtained a solid which was purified by repeated recrystallizations from methanol-CH2Cl2-di-isopropyl ether, affording a white amorphous solid that was characterized by its spectroscopic constants and by chromatographic comparison with an authentic sample as β-sitosteryl-β-D-glucopyranoside (6, 38 mg). Rf 0.31, CH2Cl2-MeOH, 9:1 [23].
Cytotoxicity Assay
The cytotoxicity of pure compounds was determined against a number of tumor cells by proliferation assay using the colorimetric method of the sulforhodamine B (SRB, protein binding dye). Cell lines of human tumors, central nervous system (U251), prostate (PC-3), leukemia (K562), colon (HCT-15), breast (MCF-7) and lung (SKLU) were provided by the National Cancer Institute (USA). Colored solutions were extracted and optical densities were read on an Ultra Reader of Microplate (Elx 808, Bio-Tek Instruments, Inc.) at wavelength of 515 nm.
X-ray crystallographic analysis of 7
X-ray structural analysis was achieved on a Bruker D8 Venture automatic diffractometer with a CCD area detector at 173(2) K using helios multilayer mirror Cu Kα radiation (λ = 1.54178Å). The structure was solved by direct methods and refined by full-matrix least-squares on F 2 using the program SHELXS-2014 (Bruker, 2014). The crystal data can summarized as follows: empirical formula C26H40O7; formula weight 464.58 amu; colorless prism, crystal system orthorhombic, crystal size 0.278 x 0.175 x 0.098 mm, space group P 21 21 21, Z = 4, a = 10.1588(2) Å, b = 10.2763(2) Å, c = 25.2894(6) Å, V = 2640.08(10) Å3, D calcd 1.171 Mg/m3; F(000) = 1012; μ = 0.681 mμ-1; 19338 collected reflections (3.49° ≤ θ ≤ 68.27°), -11<=h<=12, -12<=k<=7, -30<=l<=30; 4827 independent reflections (R int = 0.0615); goodness-of-fit on F 2 is 1.148, final R indices for I > 2 σ (I), R 1 = 0.066, wR 2 = 0.174, R indices for all data R 1 = 0.0777, wR 2 = 0.1873; refining 331 parameters and 83 restraints; the largest difference peak and hole was 0.359 and -0.232 e.Å-3; completeness to θ (25.24°) 99.8%, absorption correction was not applied. Selected crystallographic data are available as supplementary material of this article. Complete crystallographic data were deposited at the CCDC, deposition number 1432773. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html











 text new page (beta)
text new page (beta)