Introduction
Fluoroquinolones have been a class of important of synthetic antibacterial agents which are widely used in clinic for the treatment of infectious diseases [1,2]. These compounds act with an excellent activity against gram-negative and comparatively moderate against gram-positive bacteria [3-7]. Mechanism of action of these compounds is based on inhibition of an enzyme essential for bacterial DNA replication called DNA gyrase [8]. It also appears that some fluoroquinolones possess anticancer and even anti-HIV activities [9-11].
Despite there are still certain undesired events in usage of fluoroquinolones for therapeutic purposes, fluoroquinolones are one of the most important antimicrobial agents with many advantages for clinical use. Therefore there has been a growing interest in the structure modification of the fluoroquinolone skeleton and in the development of its new derivatives with increasing efficacy to prevention of hospital-acquired infections induced by fluoroquinolone-resistant pathogens [12-14]. Recent studies have shown that substituents at the 7-position of the fluoroquinolone framework highly affect their biological activity, antimicrobial spectrum, strength and target preferences [15]. For example, piperazinyl moieties substitution at this position of fluoroquinolones increase their basicity, lipophilicity and their ability to penetrate into cell walls which leads to a wide range of clinically beneficial fluoroquinolone such as ciprofloxacin, enrfoloxacin, levofloxacin, etc. [16-18].
Many synthetic protocols have been developed to accelerate the rate of amination of fluoroquinolones and to improve the yield [19-29]. Major drawbacks of these procedures include expensive reagents, use of large amounts of toxic organic solvents, prolonged heating and side reactions or using microwave. These disadvantages are not acceptable in the current pharmaceutical industry. Therefore, the development of a new greener and more convenient method for the synthesis of fluoroquinolones is highly desirable.
Giant nanosized porous Keplerate-type POMs was reported for the first time by Müller and co-workers [30]. The Keplerate and giant nanosized porous POMs show unique features which can be considered as the basis of a new type of nanochemistry and nanomaterials science [31, 32]. They find a large variety of applications in principal and applied science, such as in modelling passive cation transport through membranes, encapsulation, nanoseparation chemistry, and magnetic and optics properties [33,34].
According to the excellent acidic properties of solid polyoxometalate acids, in the last three decades, many applications as the useful and versatile acid catalysts have found in these structures [35]. Polyoxometalate acids are generally solids that are unsolvable in non-polar solvents but extremely soluble in polar ones and they can be used in both homogeneous and heterogeneous systems. Furthermore, these structures have a number of utilities involving powerful flexibility in qualification of the acid potency, easy handling, environmental friendly, non-toxicity and facile synthesis [36,37].
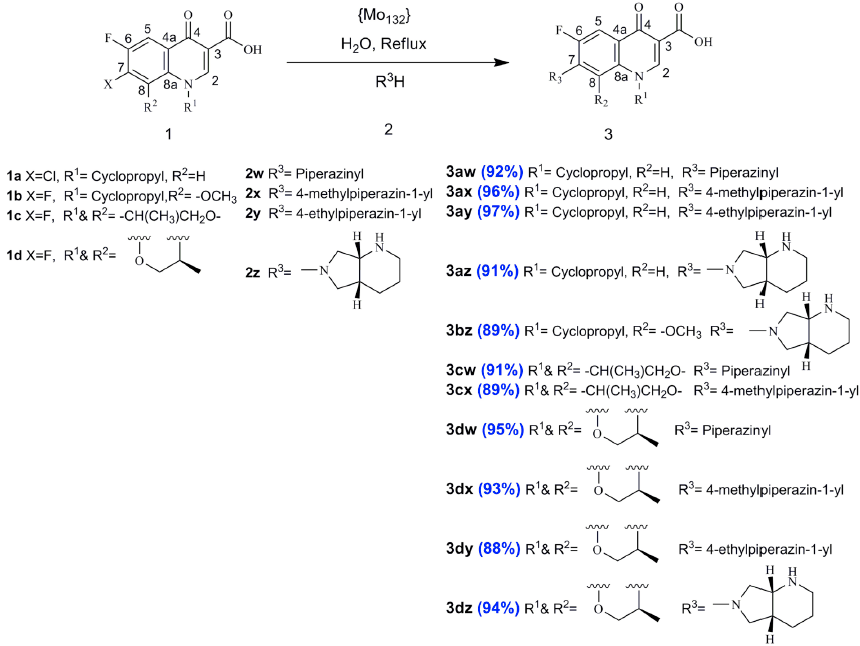
As a result of global interest in the ongoing research towards the development of environmentally friendly methods for the synthesis of organic compounds especially compounds that are frequently used in current pharmaceutical industry, we report herein facile and efficient green synthesis of fluoroquinolones as potential antibacterial with short reaction time by the two-component condensation of variety amines and some 7-halo-6-fluoroquinolone-3-carboxylic acids using a Keplerate-type giant-ball nanoporous isopolyoxomolybdate, {Mo132}, as a new catalyst with high catalytic activity under reflux condition in high yield.
In continuation of our previous works on the application of (NH4)42[MoVI 72MoV 60O372(CH3COO)30(H2O)72], a Keplerate-type giant-ball nanoporous isopolyoxomolybdate, represented as {Mo132}, as a catalyst for a series of organic transformations [38-40], we report here the application of this material as highly efficient and reusable novel catalyst to promote the reaction time, and yields of fluoroquinolone derivatives from the reaction of some 7-halo-6-fluoroquinolone carboxylic acid 1 and amine 2 in refluxing water under clean synthesis (Scheme 1). The diameter of this ball-shaped POM which calculated theoretically is 2.9 nm [31,32]. For the first time this molybdenum cluster has been characterized by the TEM image by Polarz et al. [33]. The TEM picture clearly shows a periodic structure with an average size approximately 3 nm diameter. This experimentally obtained diameter fits nicely with the theoretical value for the inner diameter of the ball-shaped POM [31, 32].
Results and discussion
The {Mo132} catalyst was characterized using FT-IR and UV-visible spectroscopies as reported in our previous work [38]. The catalytic activity of this material was evaluated in the synthesis of fluoroquinolone derivatives. At first, the synthesis of compound 3ay was selected as a model reaction to determine suitable reaction conditions. The reaction was carried out by mixture of 7-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 1a (1 mmol) and N-ethylpiperazine 2y (1.5 mmol) in the presence of different amounts of {Mo132}, and various solvents such as EtOH, H2O, MeOH, CH3CN, CH2Cl2, and also under solvent-free conditions at different temperature (Table 1). Long reaction times (>120 min) and not so good yields (45 %) of the product 3ay were obtained in the absence of the catalyst in all cases (entries 1-5). On the other hand, different amounts of the catalyst (0.02, 0.04, 0.06, 0.08, and 0.1) in the presence of the solvents or solvent-free condition in various temperatures caused to improve the yields and times of the reaction. Moreover, the best results in the presence of different amounts of catalyst were in refluxing solvents. These outcomes show that catalyst, solvent, and temperature are necessary for this reaction as well polar solvents were better than other non-polars. Also, the best yields and short reaction times were obtained in 0.08 g of the catalyst in water at different temperature (entries 12-14). Whereas, further increase in catalyst amount to 0.1 g, did not improve the product yield and reaction time (entry 15). Among the tested solvents and also solvent-free conditions and various amounts of the catalyst, the reaction was more facile and proceeded to give the highest yield (97%), and short reaction time (30 min), using 0.08 g of {Mo132} in H2O (5 ml) at reflux temperature (entry 12). All subsequent reactions were carried out in these optimized conditions.
Table 1 Optimization of reaction conditions for the synthesis of compound 3ay catalyzed by {Mo132}.*
* Reaction conditions: Ethyl 7-chloro-6-fluoroquinolone-3-carboxylic acids 1a (1 mmol) and N-ethylpiperazine 2y (1.5 mmol).
According to these results, and in order to generalize this model reaction, we developed the reaction of 1a-d with a range of various amines 2w-z under the optimized reaction conditions. The condensation of 1a-d and 2w-z afforded the products 3 in high yields over relatively short reaction times in refluxing water. The {Mo132} efficiently catalyzed the reactions, giving the desired products in high yields over relatively short reaction times. Easy separation of obtained products from the catalyst makes this method useful for the synthesis of fluoroquinolones. Purity checks with melting points, TLC, HPLC (>92%), and the 1H NMR spectroscopic data reveal that only one product is formed in all cases and no undesirable side-products are observed. The structures of all known products 3 were deduced and compared with those of authentic samples from their melting points, 1H NMR, 13C NMR, and FT-IR spectral data [18-29].
To test the recyclability of {Mo132}, after completion of the model reaction, the catalyst was recovered according to the procedure described in the experimental section. The separated catalyst was dried at 60 ºC under vacuum for 1 h before being reused in the same reaction. The catalyst could be used at least five times without significant reduction in its activity (97, 96, 94, 94, 93 % yields in first to fifth use, respectively) which clearly demonstrates the practical reusability of this catalyst.
Although we did not investigate the reaction mechanism, on the basis of our previous reports [38-40], it is reasonable to assume that several accessible Mo sites and NH4 groups in {Mo132} could act as Lewis acid and Brönsted acid centers, respectively, and therefore promote the necessary reactions. The catalyst would play a significant role in increasing the electrophilic character of the electrophiles in the reaction.
Conclusion
In conclusion, in this paper we developed the synthesis of fluoroquinolone derivatives 3aw, 3ax, 3az, 3bz, 3cw, 3cx, 3dw, 3dx, 3dy, and 3dz in the presence of {Mo132}, a Keplerate-type giant-ball nanoporous isopolyoxomolybdate, as a highly effective heterogeneous catalyst for the direct amination of 7-halo-6-fluoroquinolone-3-carboxylic acids 1a-d with several amines 2w-z in refluxing water. This method provided these products in high yields over short reaction time, following a facile work-up process. The catalyst is inexpensive and easily obtained, stable and storable, easily recycled and reused for several cycles with consistent activity.
Chemicals and apparatus
All chemicals were available commercially and used without additional purification. The catalyst was synthesized according to the literature [(32]. Melting points were recorded using a Stuart SMP3 melting point apparatus. The FT-IR spectra of the products were obtained with KBr disks, using a Tensor 27 Bruker spectrophotometer. The 1H NMR (300 MHZ) and 13C NMR (75 MHZ) spectra were recorded using Bruker spectrometers.
Typical procedure
A mixture of 7-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 1a (1 mmol) and N-ethylpiperazine 2y (1.5 mmol) and {Mo132} (0.08 g) as catalyst in H2O (5 ml) was heated under reflux for the appropriate time. The reaction was monitored by TLC. Since the catalyst solubility is very high in cold water, after completion of the transformation, the reaction mixture was allowed to cool down into room temperature. The crude product was collected by filtration, washed with H2O and recrystallized from ethanol to give desired compounds 3ay. The catalyst could be readily recovered from the combined filtrate after evaporation to dryness under reduced pressure and washing with hot ethanol.
1-Cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid (3aw)
HPLC Purity: 99.14%; Yield: 92%; 20 min; m.p: 254-256 °C (lit. [23] 255-257 °C); FT-IR (ν, cm-1 KBr disc): 3533, 3335, 3033, 2912, 1705, 1623, 1494, 1447, 1383, 1271, 1144, 1024, 804; 1H NMR (300 MHz, DMSO-d6): δ 1.15-1.20 (m, 2H, CH2), 1.30-1.35 (m, 2H, CH2), 2.90 (t, J = 6.0 Hz, 4H, 2CH2), 3.22 (t, J = 6.0 Hz, 4H, 2CH2), 3.75-3.85 (m, 1H, CH), 7.47 (d, J = 9.0 Hz, 1H, C8H), 7.75 (d, J = 15.0 Hz, 1H, C5H), 8.58 (s, 1H, C2H); 13C NMR (75 MHz, DMSO-d6): 7.9 (CH2), 36.2 (NCH), 45.8 (2NCH2), 51.1 (2NCH2), 106.9 (C3), 107.1 (C8), 111.4 (C5), 118.7 (C4a), 139.6 (C8a), 146.1 (C7), 148.2 (C2), 154.0 (C6), 165.6 (COOH), 176.6 (C4); Anal. Calc. for C17H18FN3O3 (%): C, 61.62; H, 5.48; N, 12.68. Found: C, 61.54; H, 5.37; N, 12.62.
1-Cyclopropyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-1, 4-dihydroquinoline-3-carboxylic acid (3ax)
HPLC Purity: 97.92%; Yield: 96%; 25 min; m.p: 245-247 °C (lit. [22] 248-250 °C); FT-IR (ν, cm-1 KBr disc): 3428, 3093, 2935, 1729, 1626, 1507, 1469, 1378, 1299, 1142, 1007, 885; 1H NMR (300 MHz, DMSO-d6): δ 1.17 (s, 2H, CH2), 1.32 (d, J = 9.0 Hz, 2H, CH2), 2.23 (s, 3H, NCH3), 2.20-2.35 (m, 4H, 2CH2), 3.00-3.10 (m, 4H, 2CH2), 3.75-3.85 (m, 1H, CH), 7.47 (d, J = 6.0 Hz, 1H, C8H), 7.75 (d, J = 12.0 Hz, 1H, C5H), 8.62 (s, 1H, C2H); 13C NMR (75 MHz, DMSO-d6): 8.0 (2CH2), 31.2 (NCH3), 36.3 (NCH), 45.9 (2NCH2), 49.4 (2NCH2), 106.0 (C3), 107.1 (C8), 111.0 (C5), 118.0 (C4a), 139.6 (C8a), 146.1 (C7), 148.3 (C2), 151.0 (C6), 166.3 (COOH), 176.7 (C4); Anal. Calc. for C18H20FN3O3 (%): C, 62.60; H, 5.84; N, 12.17; Found: C, 62.53; H, 5.78; N, 12.11.
1-Cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-4-oxo-1, 4-dihydroquinoline-3-carboxylic acid (3ay)
HPLC Purity: 99.06%; Yield: 97%; 30 min; m.p: 218-220 °C (lit. [22] 219-221 °C); FT-IR (ν, cm-1 KBr disc): 3533, 3335, 3033, 2912, 1738, 1627, 1470, 1381, 1337, 1254, 1154, 1022, 803; 1H NMR (300 MHz, DMSO-d6): δ 1.05 (t, J = 7.0 Hz, 3H, CH3), 1.10-1.35 (m, 4H, 2CH2), 2.42 (q, J = 6.0 Hz, 2H, NCH2), 2.50-2.60 (m, 8H, 4CH2, overlapped with solvent), 3.75-3.85 (m, 1H, CH), 7.55 (d, J = 6.0 Hz, 1H, C8H), 7.88 (d, J = 15.0 Hz, 1H, C5H), 8.65 (s, 1H, C2H), 15.23 (s br., 1H, COOH); 13C NMR (75 MHz, DMSO-d6): 8.0 (2CH2), 12.4 (CH3), 36.2 (NCH), 40.7 (NCH2), 49.8-52.4 (4NCH2), 106.5 (C3), 107.1 (C8), 111.3 (C5), 118.8 (C4a), 139.5 (C8a), 145.5 (C7), 148.1 (C2), 155.0 (C6), 166.3 (COOH), 176.5 (C4); Anal. Calc. for C19H22FN3O3 (%): C, 63.50; H, 6.17; N, 11.69; Found: C, 63.41; H, 6.09; N, 11.62.
1-Cyclopropyl-6-fluoro-7-((4aR,7aR)-hexahydro-1H-pyrrolo[3,4-b]pyridin-6(2H)-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3az)
HPLC Purity: 92.96%; Yield: 91%; 18 min; m.p: 258-260 °C (lit. 24] 256-258 °C); FT-IR (ν, cm-1 KBr disc): 3504, 3308, 3076, 2938, 1719, 1629, 1549, 1509, 1412, 1336, 1180, 1108, 888; 1H NMR (300 MHz, DMSO-d6): δ 1.10-1.35 (m, 4H, 2CH2), 1.55-1.70 (m, 4H, 2CH2), 2.50-2.60 (m, 1H, CH), 3.33 (t, J = 6.0 Hz, 2H, CH2), 3.30-3.55 (m, 4H, 2CH2), 3.63-3.75 (m, H, CH), 6.91 (d, J = 6.0 Hz, 1H, C8H), 7.65 (d, J = 15.0 Hz, 1H, C5H), 8.49 (s, 1H, C2H); Anal. Calc. for C20H22FN3O3 (%): C, 64.68; H, 5.97; N, 11.31; Found: C, 64.61; H, 5.59; N, 11.25.
1-Cyclopropyl-6-fluoro-7-((4aR,7aR)-hexahydro-1H-pyrrolo[3,4-b]pyridin-6(2H)-yl)-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (3bz)
HPLC Purity: 96.58%; Yield: 89%; 30 min, m.p: 239-241 °C (lit. [29] 238-242 °C); FT-IR (ν, cm-1 KBr disc): 3529, 3470, 3033, 2929, 1708, 1624, 1517, 1457, 1353, 1324, 1186, 1047, 805; 1H NMR (300 MHz, DMSO-d6): δ 0.81-1.25 (m, 4H, 2CH2), 1.63-1.85 (m, 4H, 2CH2), 2.60-2.70 (m, 2H, CH2), 3.10-3.20 (m, 1H, CH), 3.37 (s, 3H, OCH3), 3.60-3.65 (m, 1H, CH), 3.70-3.80 (m, 1H, CH), 3.80 -3.97 (m, 2H, CH2), 4.04-4.19 (m, 2H, CH2), 7.63 (dd, J = 12.0, 3.0 Hz, 1H, C5H), 8.64 (s, 1H, C2H), 15.15 (s br., COOH); 13C NMR (75 MHz, DMSO-d6): 8.8 (2CH2) 10.0 (CH2), 17.2 (CH2), 20.9 (CH), 34.6 (NCH2), 39.1 (NCH), 41.1 (NCH2), 41.8 (NCH), 54.4 (NCH2), 62.3 (OCH3), 106.8 (C3), 117.6 (C5), 134.9 (C4a), 137.1 (C8), 140.6 (C8a), 150.8 (C7), 151.7 (C2), 154.0 (C6), 166.3 (COOH), 176.4 (C4); Anal. Calc. for C21H24FN3O4 (%): C, 62.83; H, 6.03; N, 10.47; Found: C, 62.78; H, 5.94; N, 10.41.
9-Fluoro-3-methyl-7-oxo-10-(piperazin-1-yl)-3,7-dihydro-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid (3cw)
HPLC Purity: 95.19%; Yield: 91%; 27 min; m.p: 258-260 °C (lit. [27] 257-260 °C); FT-IR (ν, cm-1 KBr disc): 3255, 3092, 2968, 1723, 1573, 1454, 1392, 1254, 1023, 1011, 805; 1H NMR (300 MHz, DMSO-d6): δ 1.44 (d, J = 6.0 Hz, 3H, CH3), 2.80-2.85 (m, 4H, 2CH2), 3.18-3.25 (m, 4H, 2CH2, overlapped with solvent), 4.37 (d, J = 12.0 Hz, 1H, CH2, diastereotopic proton), 4.58 (d, J = 12.0 Hz, 1H, CH2, diastereotopic proton), 4.85-4.95 (m, 1H, CH), 7.51 (dd, J = 12.0, 6.0 Hz, 1H, C5H), 8.91 (s, 1H, C2H); 13C NMR (75 MHz, DMSO-d6): 18.4 (CH3), 46.6 (2NCH2), 52.0 (2NCH2), 55.2 (NCH), 68.4 (OCH2), 103.6 (C5), 107.1 (C3), 120.0 (C4a), 125.2 (C8a), 132.3 (C7), 140.5 (C8), 146.5 (C2), 154.0 (C6), 166.5 (COOH), 176.7 (C4); Anal. Calc. for C17H18FN3O4 (%): C, 58.78; H, 5.22; N, 12.10; Found: C, 58.72; H, 5.17; N, 10.36.
9-Fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-3,7-dihydro-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid (3cx)
HPLC Purity: 99.89%; Yield: 89%; 20 min; m.p: 253-255 °C (lit. [27] 250-257 °C); FT-IR (ν, cm-1 KBr disc): 3419, 3335, 3043, 2968, 1714, 1622, 1523, 1469, 1371, 1255, 1146, 1056, 804; 1H NMR (300 MHz, DMSO-d6): δ 1.44 (d, J = 9.0 Hz, 3H, CH3), 2.22 (s, 3H, NCH3), 2.35-2.50 (m, 4H, 2CH2), 3.20-3.40 (m, 4H, 2CH2), 4.35 (dd, J = 12.0, 3.0 Hz, 1H, CH2, diastereotopic proton), 4.59 (dd, J = 12.0, 3.0, 1H, CH2, diastereotopic proton), 4.85-4.98 (m, 1H, CH), 7.52 (d, J = 12.0 Hz, 1H, C5H), 8.95 (s, 1H, C2H), 15.17 (s br., 1H, COOH); 13C NMR (75 MHz, DMSO-d6): 18.4 (CH3), 46.5 (NCH3), 50.5 (2NCH2), 55.2 (2NCH2), 55.7 (NCH), 68.4 (OCH2), 103.5 (C5), 107.0 (C3), 119.8 (C4a), 125.2 (C8a), 132.5 (C7), 140.5 (C8), 146.5 (C2), 154.2 (C6), 166.5 (COOH), 176.7 (C4); Anal. Calc. for C18H20FN3O4 (%): C, 59.83; H, 5.58; N, 11.63; Found: C, 59.77; H, 5.08; N, 11.58.
(S)-9-Fluoro-3-methyl-7-oxo-10-(piperazin-1-yl)-3,7-dihydro-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid (3dw)
HPLC Purity: 99.65%; Yield: 95%; 32 min; m.p: 260-262 °C (lit. [29] 263-265 °C); FT-IR (ν, cm-1 KBr disc): 3255, 3092, 2968, 1723, 1573, 1454, 1392, 1254, 1023, 1011, 805; 1H NMR (300 MHz, DMSO-d6): δ 1.45 (d, J = 6.0 Hz, 3H, CH3), 2.75-2.85 (m, 4H, 2CH2), 3.15-3.25 (m, 4H, 2CH2, overlapped with solvent), 4.30-4.40 (m, 1H, CH2, diastereotopic proton), 4.52-4.62 (m, 1H, CH2, diastereotopic proton), 4.85-4.95 (m, 1H, CH), 7.51 (d, J = 12.0 Hz, 1H, C5H), 8.92 (s, 1H, C2H); 13C NMR (75 MHz, DMSO-d6): 18.4 (CH3), 45.8 (2NCH2), 51.0 (2NCH2), 55.2 (NCH), 68.5 (OCH2), 103.6 (C5), 107.2 (C3), 120.2 (C4a), 125.2 (C8a), 132.3 (C7), 140.5 (C8), 146.5 (C2), 154.2 (C6), 166.5 (COOH), 176.7 (C4); Anal. Calc. for C17H18FN3O4 (%): C, 58.78; H, 5.22; N, 12.10; Found: C, 58.70; H, 4.93; N, 11.51.
(S)-9-Fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-3,7-dihydro-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid (3dx)
HPLC Purity: 100%; Yield: 93%; 22 min; m.p: 225-227 °C (lit. [25] 225-226 °C); FT-IR (ν, cm-1 KBr disc): 3251, 3079, 2973, 1721, 1539, 1517, 1439, 1394, 1289, 1087, 1004, 801; 1H NMR (300 MHz, DMSO-d6): δ 1.44 (d, J = 6.0 Hz, 3H, CH3), 2.22 (s, 3H, NCH3), 2.35-2.50 (m, 4H, 2CH2), 3.20-3.30 (m, 4H, 2CH2), 4.36 (dd, J = 12.0, 3.0 Hz, 1H, CH2, diastereotopic proton), 4.59 (dd, J = 12.0, 3.0 Hz, 1H, CH2, diastereotopic proton), 4.85-4.95 (m, 1H, CH), 7.48 (d, J = 12.0 Hz, 1H, C5H), 8.94 (s, 1H, C2H), 15.15 (s br., 1H, COOH); 13C NMR (75 MHz, DMSO-d6): 18.4 (CH3), 46.5 (NCH3), 50.5 (2NCH2), 55.2 (2NCH2), 55.7 (NCH), 68.4 (OCH2), 103.8 (C5), 107 (C3), 120 (C4a), 125.2 (C8a), 132.3 (C7), 140.4 (C8), 146.5 (C2), 154.2 (C6), 166.5 (COOH), 176.7 (C4); Anal. Calc. for C18H20FN3O4 (%): C, 59.83; H, 5.58; N, 11.63; Found: C, 59.78; H, 5.50; N, 11.56.
(S)-10-(4-Ethylpiperazin-1-yl)-9-fluoro-3-methyl-7-oxo-3,7-dihydro-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid (3dy)
HPLC Purity: 99.26%; Yield: 88%; 20 min; m.p: 230-232 °C (lit. [26] 229-230 °C); FT-IR (ν, cm-1 KBr disc): 3432, 3042, 2975, 1714, 1623, 1529, 1478, 1306, 1243, 1200, 1010, 743; 1H NMR (300 MHz, DMSO-d6): δ 1.05 (t, J = 6.0 Hz, 3H, CH3), 1.45 (d, J = 9.0 Hz, 3H, CH3), 2.35-2.40 (m, 2H, CH2, overlapped with solvent), 2.40-2.60 (m, 4H, 2CH2), 3.15-3.20 (m, 4H, 2CH2), 4.37 (d, J = 12.0 Hz, 1H, CH2, diastereotopic proton), 4.57 (d, J = 9.0 Hz, 1H, CH2, diastereotopic proton), 4.91 (d, 1H, J = 6.0 Hz, CH), 7.56 (d, J = 12.0 Hz, 1H, C5H), 8.94 (s, 1H, C2H); 13C NMR (75 MHz, DMSO-d6): 12.2 (CH3), 18.4 (CH3), 46.5 (NCH2), 50.5 (2NCH2), 53.4 (2NCH2), 55.3 (NCH), 68.5 (OCH2), 103.0 (C5), 107.0 (C3), 125.2 (C4a), 126.8 (C8a), 132.3 (C7), 140.0 (C8), 146.7 (C2), 154.0 (C6), 166.5 (COOH), 176.6 (C4); Anal. Calc. for C19H22FN3O4 (%): C, 60.79; H, 5.91; N, 11.19; Found: C, 60.72; H, 5.84; N, 11.11.
(S)-9-Fluoro-10-((4aR,7aR)-hexahydro-1H-pyrrolo[3,4-b]pyridin-6(2H)-yl)-3-methyl-7-oxo-3,7-dihydro-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid (3dz)
HPLC Purity: 98.63%; Yield: 94%; 25 min; m.p: 265-267 °C (lit. [24] 265-268 °C); FT-IR (ν, cm-1 KBr disc): 3319, 3044, 2932, 1719, 1622, 1527, 1472, 1357, 1191, 1087, 1045, 862; 1H NMR (300 MHz, DMSO-d6): δ 1.30-1.70 (m, 4H, 2CH2), 1.45 (d, J = 6.0 Hz, 3H, CH3), 2.10-2.20 (m, 1H, CH), 2.80-2.90 (m, 1H, CH), 3.15-3.40 (m, 4H, 2CH2), 4.00-4.15 (m, 2H, CH2), 4.23 (d, J = 12.0 Hz, 1H, CH2, diastereotopic proton), 4.59 (d, J = 12.0 Hz, 1H, CH2, diastereotopic proton), 4.80-4.92 (m, 1H, CH), 7.47 (d, J = 15 Hz, 1H, C5H), 8.85 (s, 1H, C2H); Anal. Calc. for C20H22FN3O4 (%): C, 62.01; H, 5.72; N, 10.85; Found: C, 61.96; H, 5.74; N, 10.78.











 nueva página del texto (beta)
nueva página del texto (beta)