1. Introduction
Although nanocatalysts encompass several advantages over conventional catalyst systems [1], however, isolation and recovery of these tiny nanocatalysts from the reaction mixture is not easy [2]. To overcome this issue, the use of magnetic nanoparticles has emerged as a viable solution; their insoluble and paramagnetic nature enables easy and efficient separation of the catalysts from the reaction mixture with an external magnet [3]. Moreover, better control and understanding of the magnetic properties is now an essential tool [4, 5]. Nano-Fe 3 O 4 particles offer potential for creation of novelty in a chemical reaction, such as a Sonogashira coupling reaction, oxidation of alcohols, Munich-type reaction, and cross-coupling Suzuki-Miyaura reaction [6]. On the other hands, shorter reaction times and solvent-free condition provided by ultrasound synthesis make it ideal for rapid reaction scouting and optimization of reaction conditions [7]. Therefore, applications for sonochemistry can be found in many areas, but sonochemical processes are most widely developed for heterogeneous reactions [8].
So, combination of nano-size magnetic (Fe3O4) particles as green nanocatalyst and ultrasound irradiation provided a realistic way for synthetic organic compounds [9].
The Beckmann rearrangement of ketoximes to secondary amides is an important process step in production of organic compounds [10]. For example, Beckmann rearrangement of cyclohexanone oxime produces ε-caprolactam. ε-caprolactam is a colorless solid cyclic amide. (a lactam) with the formula (CH2)5C(O)NH. Mainly all caprolactam production goes has produced nylon 6 polymer. Nylon-6 is widely used in fibers and plastics [11, 12]. Many methods have been developed for the Beckmann rearrangement, such as Ti/montmorillanite [13], SOCl2/β-cyclodextrin [14], pivaloylchloride [15], H2SO4/Nano SiO2[16]. However, some of these methods suffer from different disadvantages such as tedious work-up procedure, drastic reaction conditions, long reaction times, undesired chemical yields and use of expensive and toxic reagents. Therefore, a milder, more selective, non-hazardous and inexpensive reagent is still required for such transformation [17].
In this article, we have arrived at a method that produces secondary amides in an ecologically responsible method, in which no aggressive reagents and non-hazardous solvents are used (Scheme 1).
2. Experimental procedure:
2.1. Chemicals and apparatus
Sonication was performed by using a Cole Palmer high intensity ultrasonic processor (600W, 20 KHz) via a micro-tip probe and 30% amplitude. Nano-Fe 3 O 4 particles and Oximes are prepared with high purity according to the reported procedures in the literature [18, 19]. Melting points were determined by Philip-Harris is melting point apparatus and are uncorrected. IR spectra were recorded on a Thermo Nicolet Nexus 670 FT-IR spectrophotometer. 1H and 13C NMR spectra were recorded on a 300 MHz Bruker Avance spectrometer in 300.13 and 75.46 MHz as CDCl3 solution. The J values are in Hertz and the chemical shifts are expressed in ppm downfield from internal TMS. XRD and SEM spectra were recorded on Philips X’pert PW3040/60 and MIRA3 TESCAN, respectively. All yields refer to isolated pure products. TLC using silica gel 60 GF254 aluminum sheet was applied for determination of the purity of substrates and products as well as monitoring the reaction.
Method, General procedure for synthesis ε-caprolactam of cyclohexanone oxime with nano Fe 3 O 4 under ultrasound irradiation:
Nano Fe3O4 (106.7gr, 0.5mol) and cyclohexanone oxime (113.2gr, 1mol) was ground in a mortar for 10 minutes, completely mixture together, then add to 10 ml H2O in a round bottom flask equipped with a magnetic stirrer. The stirred reaction mixture was irradiated with ultrasound waves at 60 oC. Sonication was continued for 45 min, and the progress of the reaction was monitored by TLC (eluent: CCl4/Et2O (5/2)). At the end of the reaction, the mixture was filtered in the presence of an efficient magnetic bar and separate nanocatalyst then extracted with diethyl ether (3×5 mL). The extracts were combined and dried over Na2SO4. After evaporation of the solvent the ε-caprolactam was obtained, in 98% yield (110.9gr, Table 2: entry 1).
TABLE 1 Optimization experiments for synthesis ε-caprolactam with nano Fe 3 O 4 under ultrasound irradiation using 1mmol of cyclohexanone oxime.
a Temperature of oil bath was 70-80 oC and irradiation with ultrasound was carried out under 30% amplitude.
b monitored by TLC.
TABLE 2 Beakmann rearrangement of ketoximes with nano Fe3O4 system.a
a All reaction carry out under ultrasound irradiation(30% amplitude).
b Molar ratio as Sub./Nano Catalyst.
c monitored by TLC.
d Determined after work-up procurer.
e Yields refer to isolated pure products from 5 g substrate.
f Lit. m.p. obtained from ref. 41-44.
TABLE 3 Deoximation of aldoximes to corresponding carbonyl compounds.a
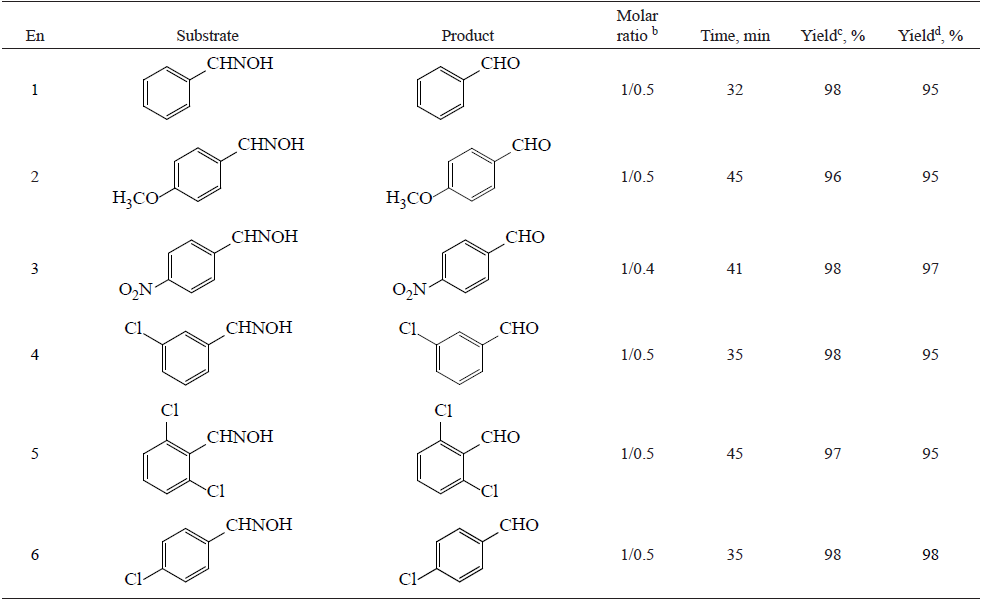
a All reaction carry out under ultrasound irradiation(30% amplitude).
b Molar ratio as Sub./Nano Catalyst.
c Determined after work-up procurer.
d Yields refer to isolated pure products from 5 g substrate.
2.2. Spectral data of selected products:
ε-caprolactam (Table2,entry1). White solid, m.p. 68-70oC. IR (KBr, ν max ): 3294, 3196, 3083, 3045, 1665, 1599, 1538, 1489, 1393, 1324, 1265, 1042, 999.9, 908, 761, 694, 534, 506 cm-1.; 1HNMR (300 MHz, CDCl3, δ ppm): 1.64-1.85 (m, 6H), 2.33-2.47 (m, 2H), 3.02 (d, 2H, J=3.9), 6.59 (S,1H).; 13C NMR (75 MHz, CDCl3, δ ppm): 23.16, 29.63, 30.56, 36.56, 42.91, 77.45, 179.34.
N-(4-Aminophenyl) acetamide (Table 2, entry3). White solid, m.p. 165oC. IR (KBr, ν max ): 3358, 3231, 3038, 1654, 1549, 1513, 1321, 1257, 1015, 834, 522 cm-1.; 1HNMR (300 MHz, CDCl3, δ ppm): 2.14 (s, 3H, CH3), 3.49 (bs, 2H, NH2), 6.64 (d, J=8.1 Hz, 2H, Ar), 6.66(s, 1H, NH), 7.26 (d, J=12, 2H, Ar) .; 13C NMR (75 MHz, CDCl3, δ ppm): 24.27, 115.40, 116.74, 122.21, 144.3, 169.
Acetanilide (Table 2, entry 2). White solid, m.p. 114oC. IR (KBr, ν max ): 3294, 1662, 1602, 1551, 1493, 755, 698 cm-1.; 1HNMR (300 MHz, CDCl3, δ ppm): 2.12 (s, 3H), 7.08 (t, 1H, J = 8.0 Hz), 7.23 (t, 2H, J = 8.0 Hz), 7.52 (d, 2H, J = 8.0 Hz), 8.54 (br. s, 1H, NH) .; 13C NMR (75 MHz, CDCl3, δ ppm): 168.9, 138.1, 128.8, 124.2, 120.2, 24.1 (CH3).
3. Results and discussion
Catalyzed organic transformations in the aqueous reaction medium are one of the ideal solutions for the development of green and sustainable protocols [20]. However, the execution of many organic reactions in water is not simply due to the inherent limitation of solubility of non-polar reactants in polar aqueous medium [21]. Which can be overcome by using ultrasound and microwave irradiation conditions [22]. Thus, when we applied nanocatalyst with ultrasound irradiation in the water medium, seems to be the perfect way to reach highly efficient chemical process.
Among nanomaterials magnetic nanoparticles are of more interest to chemical researchers owing to their praiseworthy magnetic, catalysis, photo catalysis and environmental remediation properties [23. While talking about, various magnetic nanoparticles, magnetite (Fe3O4) has been used for a several wide number of applications due to its super paramagnetic properties [24, 25].
The literature review shows that some methods such as chemical synthesis [26], thermal decomposition [27], hydrothermal synthesis [28], microwave assisted [29] synthesis is widely used to produce pure Fe3O4 particles due to its ability to control the particle size, size distribution and morphology through systematic monitoring of the reaction parameters. So we prepared Nano-Fe 3 O 4 particles with the chemical synthesis method and structure of nanoparticle elucidation was carried out by FT-IR, XRD and scanning electron microscopy (SEM).
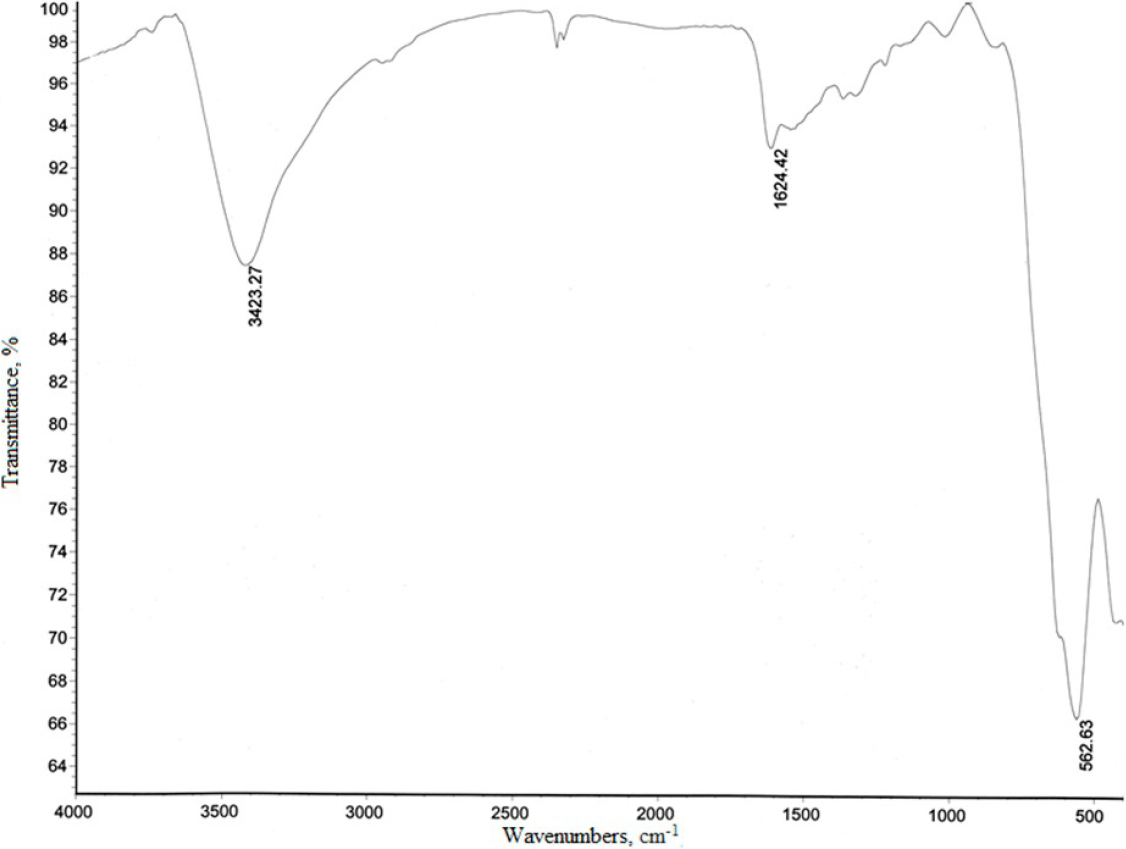
The FT-IR spectra of the synthesized Fe3O4 nano particles (Figure1) show the intense peaks at 562 cm-1 band, that is due to the stretching vibration mode associated with the metal-oxygen absorption band (Fe - O bonds in the crystalline lattice of Fe3O4) [30].
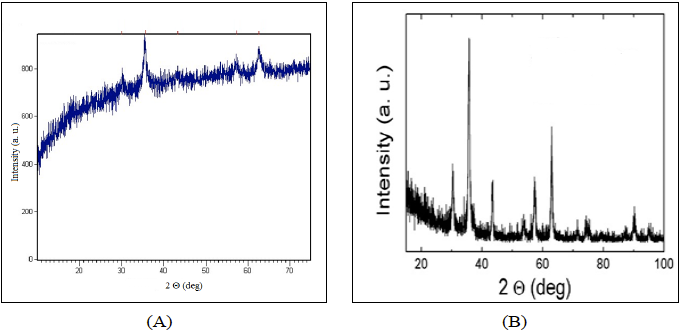
Figure 2 shows X-ray diffraction patterns of synthesized Fe3O4 nanoparticles. Comparing XRD pattern of synthesized particles with the standard diffraction spectrum (JCPDS: 65-3107), the synthesized product is crystalline Fe3O4 and the average particle size was calculated to be 16.81 nm using the Sherrer’s equation [31].
Therefore XRD particle size calculations are not quite accurate, justifying SEM observations. Corresponding SEM micrograph is shown in Figure 3, where particles are homogeneously dispersed. Particles are almost spherical with 20 nm average diameter.
In continuation of our efforts towards the development of application Nano-Fe 3 O 4 particles in organic synthesis [32] and on the other hand, ultrasound irradiation as an unconventional energy source has been widely used to perform many kinds of chemical reactions and numerous reviews and papers demonstrated its importance (33, 34, 35). We decided to synthesis secondary amides of ketoximes at present of Nano-Fe 3 O 4 particles as green nanocatalyst under ultrasound irradiation as effective method.
Although the Beckmann rearrangement is carried out with FeCl3[36] and Silferc (anhydrous FeCl3 supported on silica-gel by co-grinding method) [37], however, the reported methods suffer from the limitation in long reaction times, tedious work-up procedures.
We first optimized reaction conditions with cyclohexanone oxime compound by nano Fe3O4 under ultrasound irradiation. As shown in the experimental section, before adding H2O as a green solvent to reaction, substrate and nanocatalyst grinding in a mortar for a moment, that increase the number of collisions between OH groups of ketoximes and nanoparticle surface, that will increase the reaction rate. The results showed that using a 0.5 molar equivalent of nano Fe3O4 per molar equivalent of the oxime and 30% power amplitude of the ultrasound was the best optimum for synthesis ε-caprolactam. The reaction was completed in 45 min and ε-caprolactam was obtained in 98% yield (Table 1).
As described in Table 1, the higher yield, shorter reaction time and milder reaction condition gained under ultrasonic irradiation over in the other reaction condition present in the table (oil bath and reflux), even with a mixture of H2O(2ml)/CH3CN(2ml) for increase solve cyclohexanone oxime in solvent. that is a result of the implosive collapse of the cavitation period of the sound waves. When the formed bubbles burst, it results in high temperature and high pressure which facilitate the intermolecular reaction [38].
In routine chemical reaction applied nanocatalyst in solvent less condition, occur more efficiently and with more selectivity compared to reaction carried out in a solvent [39] (table 1, entry 4,5).)
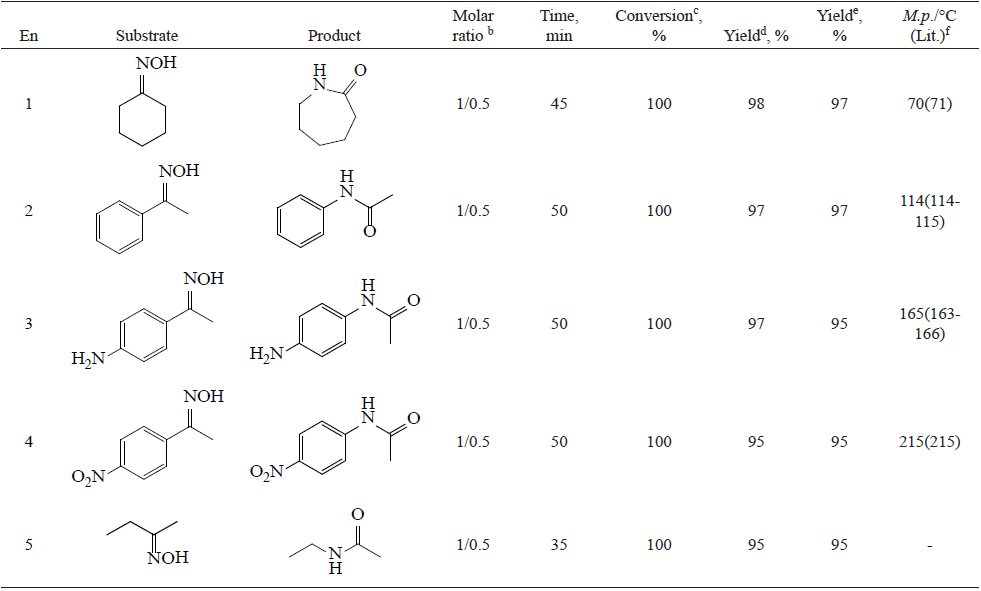
Likewise, we have applied this protocol for the rearrangement of aliphatic, aromatic ketoximes and the result summarized in Table 2. Both activated and deactivated aromatic ketoximes were converted to corresponding amides.
On examination of Table 2 some observations can be made. In all cases only one of the two possible amides were recovered. However, in case of aliphatic and cyclic systems, the reaction proceeds with quantitative conversion in reasonable time (35-45min). Moreover the results showed that migration of an alkyl group predominates over that of an aryl group (entry 5).
A case study in table 3 shows that aldoximes were deoximation completely to the corresponding carbonyl compounds under the experimental conditions.
Also for showing the advantages of this method, we applied the experiments for converting of five grams of some oxime compound used in this protocol and the result shown in the tables 2 and 3.
In continuous for application reported protocol, ketoximes selective rearrangement to the corresponding amides in the presence of aldoximes under the experimental conditions (Scheme 2).
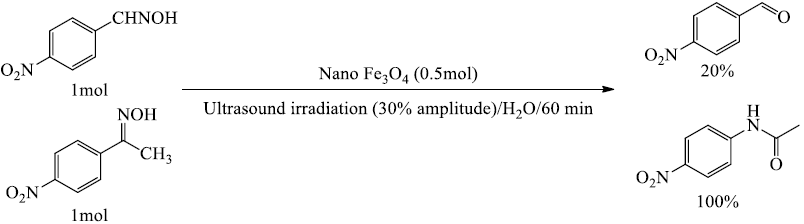
Scheme 2 Competitive reaction of aldoxime and ketoxime in the presence of nano Fe3O4 (approximately determined from TLC).
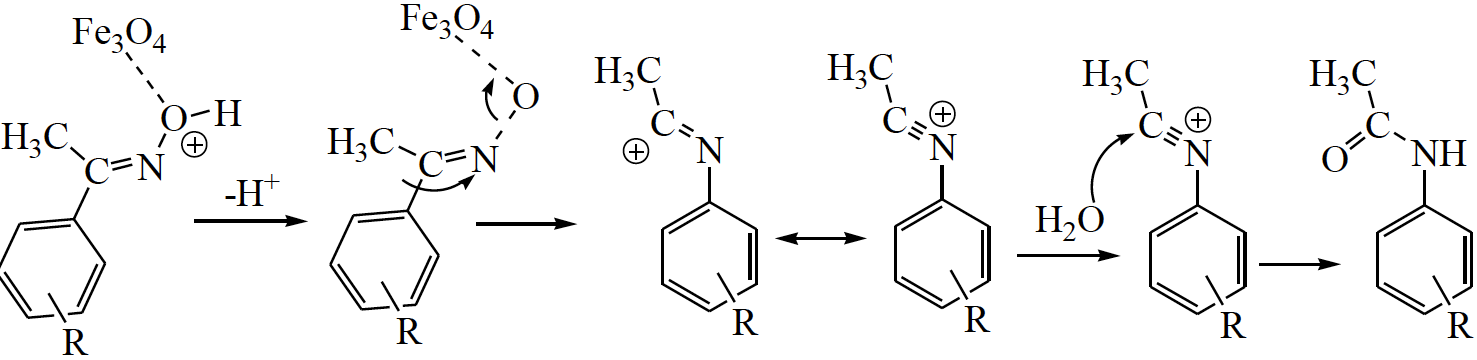
In Beckmann rearrangement hydroxyl group of a ketoxime is removed by the acidic catalyst and generate a divalent sp-hybridized azacation. Were this to occur, both carbon substitutes would be candidates for the subsequent 1, 2-shift, followed by hydrolysis, an amide is formed. The present results thus also indicate the migration of the group anti to the oxime hydroxyl group [40].
As listed in Table 2, for substrate with less than 5 grams, substrates bearing electron-donating groups in the para-position of the phenyl ring (Entry 3) were found to undergo 1,2-migration much faster than those with electron-withdrawing functionalities (Entry 4), suggesting that when an electron-donating group is present, the ring becomes more electrostatically negative, resulting in the enhancement of the migration rate, but for substrate with more than of 5 gram scale, based on the qualitative analysis by GC-MS, the cause of reducing the yield of the amide (entry 3), could be known as a byproduct (corresponding ketone).
A plausible mechanism for the Nano-Fe 3 O 4 particles catalyzed Beckmann rearrangement on ketoxime to corresponding amide was illustrated in Scheme 3. The Nano-Fe 3 O 4 particles facilitate the rearrangement process through co-ordination with - OH of ketoximes, followed by the transfer of R group.
In continuing to evaluate the recyclability and reusability of the Fe3O4 nanocatalyst, we performed the Beckmann rearrangement of cyclohexanone oximes with nanocatalyst under the optimized reaction conditions. After the completion of the reaction, the catalyst was easily separated from the reaction system and the recovered Fe3O4 nanocatalyst was washed by a sufficient amount of water and acetone. Finally, the nanocatalyst was dried in an oven at 50°C overnight before the use for next catalytic cycle, and reused for four consecutive runs, the result showed that the Fe3O4 nanocatalyst could be effectively recycled and reused up (the conversions, yield and selectivity’s were 100%, 98% and 100% for 1st run; 98%, 95% and 100% for 2nd run; 97%, 89% and 95% for 3rd run; 90%, 86% and 95% for 4th run).
4. Conclusion
In this paper, we have shown the application of Nano-Fe 3 O 4 particles efficiently catalyzed synthesis secondary amides in high yields under green solvent conditions. Simplicity, excellent yields, mildness and eco-friendly aspects of this synthetic protocol are the advantages which make this system an effective way to the present methodologies in this area.











 text new page (beta)
text new page (beta)