1. Introduction
Mining is one of the economic activities that have greatly contributed to the economic development of Mexico. The mining industry is mostly related to metal extraction and is mainly engaged in the production of copper, zinc, silver and lead. In Mexico, there have been various regions with huge mining industry such as Southern Part of the State of Chihuahua where mining activities date from 1567 [1].
In the city of Parral, in the State of Chihuahua, mining brought not only prosperity, economic growth and urban development, but also an environmental latent problem since the beginning of the operation of La Prieta mine. With an estimated production of 1,500 ton a day of pure mineral, soil wastes have been generated to date covering an area of 80 hectares approximately [2].
The town of Hidalgo del Parral is located in the south of the State of Chihuahua, between parallels 26° 51’and 27° 23’ North Latitude; the meridian 105° 23’and 105° 59’ West Longitude, and an altitude between 1,300-2,400 m. Its limits are to the north with the Municipalities of Valle de Zaragoza and Allende, to the east with the municipality of Allende, to the south with the municipalities of Allende, Matamoros, Santa Barbara and San Francisco del Oro and to the west with the municipalities of San Francisco del Oro, Huejotitán and Valle de Zaragoza. It covers 0.8% of the state’s area; it covers an area of 1,751 km2, which represents 0.71% of the state area.
Its climate is classified as semi- humid and warm, with a maximum temperature of 36 °C and a minimum of 12 °C. The annual average precipitation is 489 mm with an average of 72 rainy days and a relative humidity of 48%. The prevailing winds come from the southwest [3].
The deficiency of the mineral extraction processes used in the mid-seventeenth century [4] caused the change of methodology to selective flotation resulting in the generation of large volumes of tailings, which is estimated to have been accumulated to date up to 19,527,678 m3 ([5].
Previous studies in the area of Parral show the presence of lead and arsenic in soil (Fig. 1), exceeding the limits set by national and international organizations, which suggests that the tailings from the La Prieta mine is one of the major emission sources the metals [6, 7]. Other studies have found heavy metal contamination in water bodies in the region from mining contamination [8, 9].
In order to determine the bioavailability of the Pb and As in tailing contaminated soil, it is important to determine the chemical species of them in order to establish its mobility.
The BCR method (Community Bureau of Reference) [10] is useful to determine the bioavailability of a metal at risk of being toxic to the environment based on sequential extractions, from which each sample represents the distribution of the metal and consist in: interchangeable/soluble fraction (F1), reducible fraction (F2), oxidizable fraction (F3) and residual fraction (FR).
The aim of this study is to determine the Pb and As chemical species in soil samples in order to provide elements for policy making based on the concentration levels in Parral, Chihuahua respect to the official national stndard [11].
2. Materials and methods
2.1. Soil sampling and preparation
Sampling points were selected according to the Mexican Technical Standard Guideline [12], which establishes the specifications for the collection and handling of samples, to allow the characterization of tailings contaminated soils in the study site, through the identification and quantification of metals and metalloids, the exploratory surface sampling was designed considering a confidence interval of 95 % for a total of 19 georeferenced samples (Fig. 2). Sampling considered field measurements of parameters such as electrical conductivity, pH, temperature at 30 cm depth, relative humidity and wind speed. Transportation of tailings contaminated soil samples to the laboratory was done in low-density polyethylene bags.
Lead and arsenic total content was determined by digestion with aqua regia. One gram of sample was weighted into the reaction vessel, and then 7.5 mL of 12.0 mol L−1 HCl followed by 2.5 mL of 15.8 mol L−1 HNO3, drop by drop, to reduce foaming, was added. The temperature of the reaction mixture was slowly raised until 85°C until reflux conditions were reached and maintained for 1 h. After cooling the reaction vessel to room temperature the digests were filtered through Whatman No. 541 filter paper into 50 mL volumetric flasks, the insoluble residue onto the filter paper was washed with a 0.5 mol L−1 HNO3 and the volumetric flask was filled with 0.5 mol L-1 HNO3.
2.2. Granulometric study of samples from the site under study
A fraction of the samples, about 200 g each, were sieved for a granulometric study (Beckman Coulter LS Particle Size Analyzer). This study provides a value of the particle size which are essential because it influences in the rate of oxidation of the mineral, such that it is favored when this is finely divided, that is, the greater the surface exposed to the reaction medium if recording has fine particles that exist, it is more likely that minerals or potentially toxic elements can be released more easily to the environment, however if the particles are of larger diameter, this probability decreases slightly [13]. Some samples were selected to determine the grain diameter of mining waste present in the study area. This selection was based in points that had a higher concentration of lead and arsenic, as well as a low value (Fig. 3).
2.3. Apparatus
A Thermo Scientific (Super-Nuova Multi-Place) heating plate equipped with a thermocouple thermometer was used for aqua regia open digestion method (USEPA 3050B). Lead and arsenic were determined in digests and extracts using a Perkin Elmer 3100A spectrometer and a Perkin Elmer Analyst 100 spectrometer equipped with a MHS-10 hydride system.
2.4. Standards and Reagents
All the solutions were prepared with de-ionized water 18 MΩ·cm, Millipore Milli-Q system. Arsenic and lead stock solution contains 1000 μg/mL (High-Purity Standards). These stock solutions were kept at 4 °C in darkness. More dilute solutions for the analysis were prepared daily for each analysis.
Nitric acid (HNO3, Baker, Instra-analysed, 70%) and hydrochloric acid (HCl, Baker, Instra-analysed, 36.5-38%) were used for the aqua regia digestion.
2.5. Storage and preparation of soils samples for total concentration analysis
Sample preparation involves the following steps: (1) drying, (2) homogenizing and sieving, and (3) storage. The samples were digested with a mixture of aqua regia (nitric acid: hydrochloric acid in 1:3) [14].
2.6. Analytical methods
Lead and Arsenic concentrations in digests and extracts were determined using Flame Atomic Absorption Spectroscopy and Hydride Generation-Atomic Absorption Spectroscopy respectively (15, 16). For the measurement of Pb and As, hollow cathode lamps operated at 10 mA and a 283 nm wavelength, and 19 mA and a 193.7 nm wavelength were used respectively; the spectral band was 0.7 nm for Pb and 2.0 nm for As. Concentration of metals was obtained by the standard calibration technique.
To calculate the limit of detection (LOD) and the limit of quantification (LOQ), three curves were prepared, obtaining the standard deviation of the intercept (Sb) and average of the slope. To evaluate the linear range, a calibration curve was prepared in the concentration range of 5 to 50 ng/L for arsenic and 5 to 30 mg/L for lead. Calibration sensitivity was determined by using the slope of the calibration curve. The accuracy of the method was evaluated as percentage of recovery by adding known amounts of arsenic and lead standard before the process of digestion and extraction to a blank sample. All samples were analyzed in triplicate. The results are expressed as mean values with standard deviations (SD) as a measure of dispersion (average ± SD).
2.7. Sequential Extraction Procedure
The sequential extraction was carried out according to the BCR (Community Bureau of Reference of the European Community) three-step procedure [10]. The method determines four well defined geochemical fractions of metals in soils and sediments: acid-soluble/exchangeable fraction (F1), reducible fraction (F2), oxidizable fraction (F3), and residual fraction (RF) [17]. Extractions were performed using the reagents given in section 2.4.
Extracting reagents were: acetic acid (CH3CO2H Sigma-Aldrich 99.7%), hydroxylamine hydrochloride (NH2-OH HCl J. T. Baker), hydrogen peroxide (H2O2 J.T. Baker 30%) and ammonium acetate (CH3CO2NH4 J.T. Baker). Hydride-generating reagents were: sodium borohydride (NaBH4 tablets, Fluka, purity >97% at 3% in 1% NaOH); HCl 15% were used for pre-reduction in the determination of arsenic after aqua regia leaching.
Step 1 (acid extractable/exchange fraction-bound to carbonates):
A total of 40 mL of acetic acid 0.11 mol L-1 was added to 0.5 g air-dried sample and shaken for 16 h. The mixture was centrifuged to separate the extract from the residue using a centrifuge (Labnet Hermle) at 3000 rpm for 15 min and the supernatant solution filtered through Whatman No. 40 filter paper, rinsed with deionized water, hand shaken and stored in acid rinsed polypropylene tubes for further analysis. The residue was washed with 20 mL of distilled water by shaking for 20 min, centrifuged and the washings discarded.
Step 2 (reducible fraction-bound to Fe/Mn oxides):
A total of 40 mL of hydroxylammonium chloride 0.5 mol L-1, adjusted with nitric acid to pH 2, was added to the residue from step 1 and the extraction performed as above.
Step 3 (oxidizable fraction-bound to organic matters and sulfides):
The residue from step 2 was treated twice with 8.8 mol L-1 hydrogen peroxide, first digestion at room temperature (approx. 23±2 ° C) for 1 h and a second digestion for additional 1 h at 85±2 °C in a water bath, then 50 mL of ammonium acetate 1 mol L-1, was added and the extraction performed as step 1, the solid residue was retained for aqua regia digestion.
Step 4 (residual fraction):
Residual material remaining after step 3 of the sequential extraction was digested in the same manner after quantitative transferring of the residual from PTFE vessel to the glass flask. The residue from the last step was digested with a mixture of 20 mL aqua regia as described above.
Using the computer program SURFER, the data were introduced to develop a map of iso-concentration for observe in general the situation of the sampling points with higher concentration of arsenic and lead in the study area.
3. Results and discussion
During the fieldwork, statistical data of environmental parameters were listed in Table 1, the observed.
3.1. Granulometric study
The results of the sieve study for the selected samples is summarized in Table 2, as seen the averages ranging between 154 microns and 379 microns, with an overall median of 266.94 microns and a standard deviation of 81.32 microns.
3.2. Total concentration of arsenic and lead in soil samples
For the determination of the total lead and arsenic concentrations in the digested samples the calibration curve was in the range aforementioned (r = 0.997, n = 6 for arsenic and r=0.998, n=6 for lead). The LOD was 0.01 mg kg−1 and 0.13 mg kg-1 and the LOQ was 0.03 mg kg−1 and 1.13 mg kg−1 respectively. The percentage of recovery showed no significant differences between the measured values for the total arsenic and lead content in the blank sample.
Total concentration of arsenic and lead (mg/kg dry material) from the total digestion analysis is presented in Table 3.
Table 3 Total concentration of As and Pb (mg/kg dry weight) in soil samples from Hidalgo del Parral, Chihuahua
a The sampling point was removed due to difficult access to the study site.
bBackground soil.
In the case of arsenic concentrations ranged from 7.42 ± 0.65 to 509.84 ± 40.18 mg kg-1, 8 soil samples presented total As concentrations higher than 260 mg kg-1, which has been used by Mexican guidelines as trigger level for industrial soil remediation [11]. In detail, 47% of soil samples presented arsenic concentrations between 299-510 mg kg-1 (HP1, HP5- HP7, HP12, HP13, HP17, HP18) and the other 53% of samples are below 207 mg kg-1 (HP2-HP4, HP8-HP11, HP14-16). The reported arsenic is originated by the mining activities, this is reflected because the concentration of the background soil which contains 10.44 ± 0.83 mg kg-1 of arsenic. Figure 4 shows that the concentrations of As correspond to the samples taken in the northern part and the south part of the study area, this areas correspond to the mining tailings that are near to the residential zone.
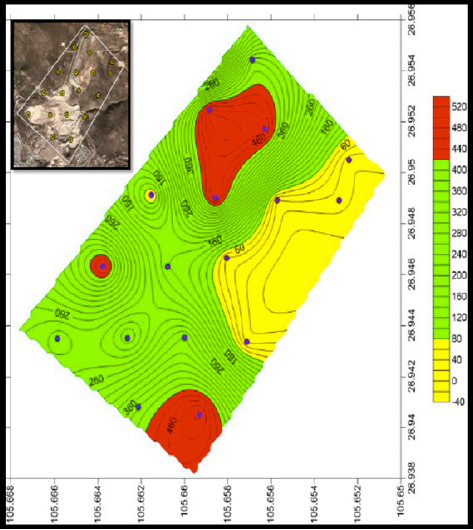
Figure 4 Overview of total arsenic concentrations of sampling points in the study area in mg/kg (SURFER).
In the case of lead concentrations ranged from 78.03 ± 2.67 to 5748.24 ± 263.63 mg kg-1, 13 soil samples presented total Pb concentrations higher than 800 mg kg-1, which has been used by Mexican guidelines as trigger level for industrial soil remediation [11]. In detail, 68% of soil samples presented lead concentrations between 1194-5748 mg kg-1 (HP1, HP2, HP3, HP4, HP5, HP6, HP7, HP9, HP12, HP13, HP16, HP17, HP18) and the other 32% of samples are below 711 mg kg-1 (HP8, HP10, HP11, HP14, HP15). The reported lead is originated by the mining activities, this is reflected because the concentration of the background soil which contains 78.03 ± 2.67 mg kg-1 of lead. Figure 5 shows that the concentrations of Pb correspond to the samples taken in all the study area, which is near to the residential zone.
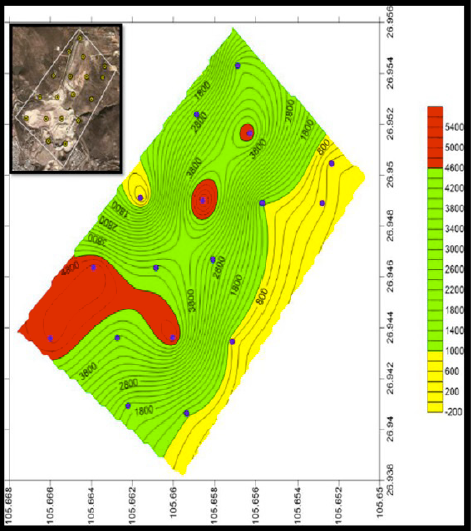
Figure 5 Overview of total lead concentrations of sampling points in the study area in mg/kg (SURFER).
It should be noted that the prevailing winds direction (from SO to NE) [18] in the area also pose a risk to the population, growing areas near the mine.
3.3. Geochemical fraction of As and Pb
Heavy metal mobility and bioavailability in soils depend strongly on the mineralogical and chemical forms in which they occur. The results (Table 4) of this investigation have shown that for arsenic were highly associated with the oxidizable (F3) and residual fraction (FR), for arsenic. Almost 90% of the samples have most of the arsenic in the residual fraction (RF), these fraction is characterized by low mobility and low probabilities of posing adverse effects to the environment. It’s observed that the content of arsenic in fraction 2 (F2) have less than 50 mg kg-1 of arsenic, although these values are minimal in a matter of accessibility, the content of arsenic in this zones is higher than the values established at international (0.39 mg/kg for US residential areas, 12 mg/kg for Canada, 32 mg/kg for United Kingdom) and national guidelines (22 mg/kg for residential/agricultural soil) [11, 19, 20]. That exposure to arsenic poses a carcinogenic hazard to the lung via both the oral and inhalation routes of exposure. The sites require more attention due to the possible risk to potentially exposed residents. As it can be observed in the Table 4, the samples have shown insignificant amounts associated with the extractable/exchangeable fraction (F1).
Table 4 Distribution of element fractionation for arsenic (mg/kg weight dry) in the study area of Hidalgo del Parral
a= [(F1+F2+F3+TR)/ (Total digestion)]×100
For lead (Table 5), distribution varies depending on the sample; it has no standard behavior as the case of arsenic. In detail 8 samples (HP1, HP2, HP5, HP6, HP9, HP13, HP17, HP18) contain more than 70% of lead in fraction 1 (F1) and fraction 2 (F2). The F1 involves weakly adsorbed metals retained on the solid surface by relatively weak electrostatic interaction; metals that can be released by ion-exchangeable processes. Metals on this fraction also can be replaced by neutral salts. The second fraction, bound to iron and manganese oxyhydroxides that are present in the soils can change their adsorption capacities drastically according to the redox conditions (presence/absence of O2), producing either FeS or FeO (OH) liberating co-precipitated or adsorbed metals at every change [21]. For the rest of the samples, lead is distributed in the second and third fraction (F2 and F3). Given the absence of organic matter in the case of F3, the extracts obtained during this step are metals bound to sulphides, being unstable only under severe oxidizing conditions [22], making it one of the most stable fractions. As it is observed, lead concentrations in the F1 are above the values established at international (840 mg/kg for US residential areas, 750 mg/kg for European Union) and national guidelines (400 mg/kg for residential/agricultural soil) [11, 22].
Table 5 Distribution of element fractionation for lead (mg/kg weight dry) in the study area of Hidalgo del Parral
a= [(F1+F2+F3+TR)/ (Total digestion)]×100
For both elements the sum of concentrations for each fraction was compared with the total concentration from the values in Table 3. Reasonable percentages of recovery of all samples for arsenic were from 51 to 76 %. It is considered that solubility and equilibration limitations in extraction procedure are probably responsible for the lower extractability of arsenic [23]. In the case of lead [24] good recovery percentages are observed ranged from 85-112%. Less stable forms of arsenic and lead compounds ranged between 1.84-48.99 and 3.29-2,538 mg kg-1 respectively, which indicated a potential risk.
3.4. Risk estimation from exposure to bioaccessible fractions
Regarding the doses of reference published in the Integrated Risk Information System [25] of the United States for As (3 x 10-4 mg/kg-d for oral exposure), and considering an ingestion of 32 mg soil/d [26], through a hand to mouth mechanism and considering a mean weight in Mexican male population of 77.7 kg [27], there is low potential risk to the population of Parral since the exposure is from 15 to 396 times smaller than the dose of reference. However, there is risk cancer through inhalation. Risk calculation could not be done for Pb since there is no dose of reference available. On the other hand, a mixed effect of As and Pb exposure might be assessed.
3.5. Policy Making Proposal
According to the Mexican Ministry of Environment [28], there are more than 635 potentially contaminated sites in Mexico. From those, just a little fraction has been remediated because of limited funding and limited capacity for characterizing and lack of a methodology to prioritize them. Even though there is a standard for contaminated soil since 2007 [11], there was not considered a clear assessment for stability of chemical species in soil matrixes. This situation is similar in other Latin American Countries and other regions where the lack of regulation has turned into large impacts of mining activities.
Chemical speciation methods can provide a set of criteria that might be used by local authorities to prioritize contaminated sites and focus efforts in those with higher risk to health or the environment. Fractions F1 and F2 are the ones that might be of concern while combined with high exposition through ingestion or respiration.
4. Conclusions and recommendations
An exploratory and systematic surface sampling was done in order to establish the presence of arsenic and lead in the mining area of Hidalgo del Parral, Chihuahua.
The arsenic and lead quantification method was validated using Atomic Absorption Flame & Hydride-Generation Spectrometry with appropriate performance and finding concentration ranges of 7.42 ± 0.65 to 509.84 ± 40.18 [mg kg-1] for arsenic & 83.34 ± 2.67 to 5579 ± [mg kg-1] for lead.
From a total of 18 samples taken at the site, eight samples (47%) had higher concentration of arsenic them the limit established in NOM-147-SEMARNAT/SSA1-2004, which corresponds to 260 mg kg-1; on the other hand, 13 samples (76%) had higher concentration of lead than the limit of 800 mg kg-1 established in the same regulation. The samples out of range for both metals were taken to a speciation test consisting in sequential extractions using the method BCR (Community Bureau of Reference) to determine their bioavailability.
In case of arsenic, the speciation shows that the higher fraction is in the oxidized fraction (F3) and in the residual fraction (FR), which suggest that the mobility & bioavailability of this element is very low as well as the migration to other compartments; therefore, environmental & health effects are not evident. However, even though the arsenic concentration in the reducible fraction (F2) is lower than 50 mg kg-1, the proximity to populated areas increases the chronic exposition risk from ingestion/respiration of dusts in these sites.
In case of lead, eight samples had the higher concentration in the fraction F1 and fraction F2, suggesting that the little stability of the metal is high since the chemical species have weak adherence to the soil matrix and can be released by ionic exchange processes; these samples had concentration values higher than the limit of the soil standard and represent a risk to health and the environment.
For both elements, recovery percentage of the speciation method was evaluated comparing the results with the total concentration of the samples, showing values of 85% to 112%.
Regarding the risk quantification for arsenic exposure, dose of reference was not reached by two orders of magnitude. However, there is risk of a mixed effect of Pb and As chronic exposure.
A bioaccesibility study for both metals as well as a fine particle (PM2.5) in air are recommended in order to assess urban contamination and the risk to health by accidental ingestion of metal-polluted soils.
As Policy Making action, risk assessment should be included in environmental regulation using the estimation of chemical speciation of species of concern (those that are more easy to move in the environment and that can be absorbed by the organism).











 nueva página del texto (beta)
nueva página del texto (beta)