Introduction
Xanthenes and benzoxanthenes are an important class of organic compounds. Xanthenes and its derivatives are important in the area of medicinal chemistry. They have various pharmacological activities such as antibacterial [1], antiviral [2] and anti-inflammatory properties [3]. Furthermore, they have been utilized as antagonists for drug-resistant leukemia lines [4] and in photodynamic therapy [5]. They are also applied as dyes in laser technology [6] and as pH sensitive fluorescent materials for visualization of biomolecules [7].
For the construction of xanthenes and benzoxanthenes, various procedures are available including the cycloacylation of carbamates [8], cyclocondensation between 2-hydroxy aromatic aldehydes and 2-tetralone [9], cyclodehydrations [10] and intramolecular phenyl carbonyl reaction of aldehydes with β-naphthol [11]. However, these methods suffer from certain drawbacks including long reaction times, and using poisonous and expensive catalyst materials. Some of the catalysts are destroyed in the work-up procedure and cannot be recovered or reused. Therefore, the search continues for a better catalyst to synthesize of benzoxanthenes in terms of operational simplicity, reusability of catalyst, low cost, and greater selectivity.
Recently, mesoporous molecular sieves (like MCM-41 and MCM-48), have attracted much interest in catalysis, due to their high surface area, well defined pore shape, narrow pore size distribution, and good thermal stability. These are particularly attractive for heterogeneous reactions of large organic molecules for which microporous zeolites cannot be used. These mesoporous material are prepared by using ionic surfactants [12]. Generally, the residual template inside the pores is removed by calcination or extraction. As ionic surfactants contain an organic cation such as cetyltrimethylammonium (C19H42N+) and an inorganic anion, we hypothesized that they could act as an ionic liquid. Herein, we report the preparation of two xanthene derivatives in the presence of template-containing Zn/MCM-41 as heterogeneous catalyst under solvent free conditions.
Results and discussion
The low angle XRD patterns of MCM-41 exhibit four well-resolved peaks with a very intense diffraction peak (100) at 2θ ≈2.5 and three peaks with lower intensity at ca. 3-5o which were indexed to the 110, 200, and 210 planes characteristic for textural uniformity of the hexagonal p6mm structure [13]. All Zn/ MCM-41 samples show a typical peak about 2θ = 2.2o, the characteristic peak of MCM-41, indicating that the ordered mesoporous structure of parent MCM-41 was preserved after the introduction of zinc ions (Fig. 1). However the catalyst prepared by the WI method does not have long-range crystalline order because the reflections due to higher indices became weak.
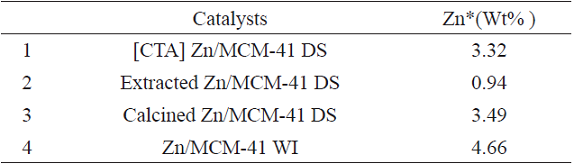
The elemental concentration distribution on the catalyst is reported in Table 1. EDX results showed that using the WI method high metal loading can be attained, because there is no intermediate washing step involved in wet impregnation approach, in contrast to the direct synthesis procedure. Furthermore the solvent extraction method decreases the metal loading because during this process, the zinc ions leach out of the support.
3.1 Template removal
In general, the template molecules are burned off by calcination at 500-600°C in air or oxygen. An alternative method for template removal is to extract it with hot conventional solvent. In this work, the organic template molecule was removed from the silicate lattice with these two techniques.
The removal of the template from as-synthesized MCM-41 was confirmed by Infrared spectrophotometry. Fig. 2 shows the FTIR spectra of template-containing Zn/MCM-41, solvent extracted and calcined Zn/MCM-41 samples. The [CTA]Zn/MCM-41 sample exhibits absorption bands around 2970 cm-1, corresponding to C-H stretch vibrations of the surfactant molecules. Two weak peaks in this region are also observed in the spectrum of solvent extracted Zn/MCM-41 samples. This was probably caused by the incomplete extraction of the template. While the traditional calcination method fully eliminates the template, solvent extraction always leaves some traces of surfactant.

Fig. 2 FT-IR spectrum of a) [CTA] Zn/MCM-41 , b) Ethanol extracted Zn/MCM-41, c) Calcined Zn/MCM-41 prepared by direct synthesis.
In our previous work, TGA analysis was also employed to determine quantitatively the amount of surfactant [14]. The amount of template was estimated from the weight loss between 150 and 450°C. These amounts were approximately 25%, 5% and lower than 0.5% for template-containing, extracted, and calcinated Ca/MCM-41, respectively. These data also confirmed that most of the template molecules had been released via calcination.
3.2 Synthesis of xanthene derivatives
There are many reports for the synthesis of xanthene derivatives. The highest yield has been obtained by an ionic liquid as catalyst [15,16]. However there are certain concerns over the use of ionic liquids as green solvents because large amounts of organic solvent and energy are used in their preparation [17]. As we believed that the ionic surfactant could act as an ionic liquid, we compare the catalytic activity of template-containing and free template Zn/MCM-41 catalysts in a one-pot multicomponent condensation. Table 2 shows the catalytic performance of prepared catalysts in the reaction between benzaldehyde and β-naphtol in the presence of dimedone. It was expected that the reaction yield would increase with the zinc content of the catalyst, in accordance with an increase in the concentration of Lewis acidic centers. Furthermore, compared to calcined catalyst and catalyst prepared by wet impregnation, the template-containing Zn/MCM-41 exhibits superior catalytic activity in terms of yield (82%), and reaction time (Table 1, entry 1, 3, 4). Actually, the cationic surfactant CTAB accelerated the model reaction to afford the desired product in good yield. The higher activity of extracted Zn/MCM-41 with very low zinc content, than the corresponding calcined form, confirmed this effect (entry 2 & 3), because extracted catalyst still contained a low amount of the template. On the other hand, metal free [CTA]MCM-41 showed lower activity in comparison to [CTA] Zn/MCM-41. It can be concluded that ionic surfactant intensifies the effect of Lewis acid sites.
Table 2 Synthesis of compound 1 using Zn incorporated MCM-41 catalystsa

a Reaction condition: dimedone(1 mmol), β -naphtol (1mmol) and benzaldehyde (1mmol) ; 0.1gr catalyst
b Isolated yield
To prove the complementary effect of ionic surfactant, the catalytic activity of prepared Zn/MCM-41 catalysts was also evaluated in the synthesis of a hydrobenzene derivative from dimedone and benzaldehyde (Table 3). Interestingly, template-containing Zn/MCM-41 exhibited high catalytic activity for the synthesis of 3,3,6,6-tetramethyl-9-phenyl-1,8-dioxooctahydro xanthene (entry 1).
Table 3 Synthesis of compound 2 using Zn incorporated MCM-41 catalysts a
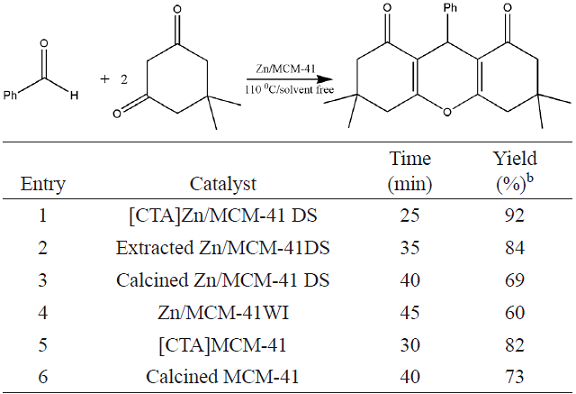
a Reaction condition: dimedone(2 mmol) and benzaldehyde (1mmol); 0.1gr catalyst
b Isolated yield
Ionic surfactant enhances the rate of this kind of reactions because the charged intermediates are present. Moreover it increases effective collisions between reactants. Generally, ionic surfactants in hydrophobic substrates can self-assemble reverse clusters, which provide a large interface between the catalyst and reactant where the reaction takes place, so greatly enhancing the reaction rate [18]. Also, the formation of CTAB cluster would congregate catalytic species Zn2+ at the reaction interface, which could catalyze the reaction more efficiently.
3.4 Reusability of catalyst
One of the most important features of a solid catalyst is the ability to be recycled. The template-containing Zn/MCM-41 synthesized via direct approach ([CTA]Zn/MCM-41 DS) that displayed the best catalytic activity was studied further. After the first test, the used catalyst was filtered, washed with ethyl acetate to remove any unreacted precursor and organic products. Then the recovered catalyst was charged for the next run. Recyclability of the catalyst was assessed by using it for 5 cycles (Table 4).
EDX analysis was performed to estimate the Zn content of the recovered catalyst after first and fifth run showing the presence of Zn atomic 3.09 and 2.86 respectively. This indicates that negligible leaching of the zinc from this support occurs under the reaction condition. So it has substantial stability under the used experimental conditions. Furthermore, XRD patterns of recovered Zn/MCM-41 catalysts showed the arrangement of mesoporous structure of MCM-41 was retained after reaction (see supplementary data, Fig. S5).
To determine the amount of organic template (CTA) that remained in the pores of the recovered [CTA]Zn/MCM-41, a thermogravimetric analysis experiment was performed. The TG curve of the as-synthesized Zn/MCM-41 DS is shown in Fig. 3a. Three distinct stages of weight loss were observed in template-containing Zn/MCM-41, weight loss below 150 °C (due to desorption of water), between 200-450 °C (due to decomposition of the template) and above 450 °C (due to water loss via condensation of silanol groups to form siloxane bonds). Weight loss of about 22 % at temperatures between 200-450 °C was observed. This amount was approximately 21 % for [CTA] Zn/MCM-41 after fifth run (Fig. 3b). So despite a weak interaction of CTA cations with siloxy anions, they are not continuously leached because they are stabilized in the micelles as a consequence of a strong interaction of the non-polar tails [19,20]. Therefore, [CTA]Zn/MCM-41 was found to be a reusable catalyst, which is one of the main requirements for employing this catalyst for the production of xanthenes derivatives.
Conclusions
Zn/MCM-41 catalysts have been synthesized by direct synthesis and wet impregnation methods. Then the ionic template was removed by two different methods: solvent extraction and calcination. The prepared samples were used as catalyst in the synthesis of two xanthene derivatives. The results showed that template-containing Zn/MCM-41, prepared by direct synthesis approach, was the most active catalyst for this reaction, which could be attributed to the Lewis acid sites and ionic template effect. This catalyst was reused without significant loss of activity. Since there is no need to remove the template, this strategy saves energy and time and it also reduces the pollutants released to the environment. Furthermore, this catalyst is synthesized by a one-pot approach via a non-hydrothermally simple method and is cost effective.
Experimental Section
Cetyltrimethylammonium bromide (CTAB) was purchased from Sigma-Aldrich Inc. and the other reagents were purchased from Merck Company.
Catalyst preparation
-Direct SynthesisMethod (DS)
Zn/MCM-41 samples were synthesized according to standard literature procedure [21]. In a typical non-hydrothermal synthesis, 2.74 mmol of CTAB were dissolved in 480 mL of NaOH aqueous solution (15.0 mM), followed by a drop wise addition of 22.4 mmol of tetraethylorthosilicate (TEOS). Then, a solid powder of zinc nitrate (0.18 g) was slowly added. The mixture was vigorously stirred and heated to 80 °C for 2 h. Subsequently, the product powder was isolated by hot filtration, washed with deionized water and dried in air (at 25°C).
In some preparations the cationic surfactant was removed either by hot solvent extraction or by calcination in air at 640 °C for 6 h. The solvent extraction was performed by stirring 1 g of the air-dried product with a 1 M HCl solution in ethanol (liquid: solid 300 mL/g) at 333 K for 24 h. Then the mesoporous material was collected by filtration and dried at room temperature. In order to compare the effect of the template, a series of as-synthesized molecular sieves, containing their organic template, were also tested for benzoxanthene synthesis without further modification.
-Wet Impregnation Method (WI)
The calcined MCM-41 sample was introduced into a calculated amount of Zn(NO3)3.6H2O aqueous solution (0.18 g). After 3 h, the resultant mixture was dried at 60 °C.
Catalyst characterization
XRD measurements were performed on a Philips-PW 17C diffractometer with
Synthesis of 12-phenyl-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[
A mixture of β-naphthol (0.144 g, 1 mmol), dimedone (0.140 g, 1 mmol), benzaldehyde (1ml, 1 mmol) and Zn/MCM-41 (0.1 gr), was stirred in an oil-bath (110 °C) [22]. After completion of the reaction, as monitored by TLC, the reaction mixture was cooled to room temperature; ethyl acetate (15 mL) was added to it, and stirred for 10 min. Then the catalyst was separated by filtration. Finally, ethyl acetate was evaporated under vacuum to give the crude product. The crude product was recrystallized from EtOH to yield pure xanthenes-11-one derivatives.
All pure products were characterized by comparison of their physical (melting point) and spectral data (1H NMR) with authentic samples (supplementary information).











 nueva página del texto (beta)
nueva página del texto (beta)