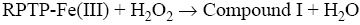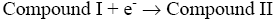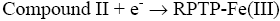Introduction
The rapid and sensitive detection of hydrogen peroxide (H2O2) is a major area of interest within the fields of: environmental control, chemical and pharmaceutical industry as well as clinical diagnostics [1]. Different analytical techniques have been employed in order to detect H2O2, among the most widely used techniques are: UV-vis spectroscopy, chemiluminescence and titration methods [2, 3]. Despite these methods being effective in the detection of H2O2, the requirement of sample pretreatment, bulky instruments and longtime analysis open the space for new analytical methods where the detection could be done in a direct and faster way. Such new detection method offering direct sample analysis, faster response and possibility of miniaturization will accelerate the use of H2O2 detection on-site and its integration in for instance lab-on-a-chip devices.
Electrochemical biosensors based on plant peroxidases have attracted special attention due to their simplicity, rapidity, possibility of miniaturization and convenience for detection of several analytes [4, 5]. Horseradish peroxidase (HRP) is a redox enzyme with a pI 8.8 that belongs to class III of the plant peroxidase superfamily. Traditionally it has been the most commonly used oxidoreductase for the development of enzyme-based amperometric biosensors [6]. HRP has a very broad specificity towards amines and phenolic compounds and good sta bility at room temperature, however the main disadvantage is its low stability towards high concentrations of H2O2 and other hydroperoxides [7]. These drawbacks have been a motivation for the search of new sources of plant peroxidases with higher stability and different specificity.
Recently a new plant peroxidase was found on the leaves of Royal palm tree [8]. The main attraction of the Royal palm tree peroxidase (RPTP) lies on its stability to high concentrations of H2O2 and to extreme pHs and temperature [8]. RPTP is extracted from the leaves of Royal palm tree and comprise a single polypeptide chain, about 300 residues in length, two Ca2+ atoms, and a heme prosthetic group. The substrate specificity toward phenols and amines is similar to other plant peroxidases making this enzyme a potential biocatalyst for commercial and environmental applications. Csöregi et al. [5] studied the comparison of plant peroxidases for the construction of electrochemical biosensors and it was found that soybean peroxidase immobilized on graphite electrodes (SPP) was the most efficient peroxidase for H2O2 detection. In a similar approach Sakharov et al. [6] demonstrated the high stability of anionic RPTP immobilized on graphite electrodes for detecting H2O2. However the electrochemical and microscopic characterization of the electrode surface in the previous report was incomplete, in this paper a deeper voltamperometric study of RPTP is warranted.
In recent years, researchers have shown an increase interest in a special class of carbon nanomaterials for the construction of biosensors in order to detect molecules of biomedical and environmental interest [9]. Since it was reported in 2004, graphene has been attracting a lot of attention as a new nanomaterial with interesting electronic and electrochemical properties for their use in the development of electronic detection devices. More and more attention has been given to the functionalization of graphene with biomolecules for their use in fields such as biosensing. Enzymes, antibodies, metal nanoparticles, DNA and quantum-dots are some of the materials used for the functionalization of graphene [9].
Most of electrochemical studies has focus on conventional graphite [10], glassy carbon [11], platinum [12] and gold [13] electrodes. Despite of its efficiency and quality, the high cost and laborious pre-treatment limit their commercial application. Screen printed electrodes (SPE) are transduction elements that combine ease of use, portability and inexpensive manufacture procedure thereby facilitating mass production for using as a single electrodes and biosensors.
To the best of our knowledge, not previous studies has investigated about the fabrication and evaluation of a SPGE modified electrode with the stable RPTP immobilized in chitosan-glutaraldehyde. In this study we obtain conjugates formed by CS-GA-RPTP to be integrated into a graphene electrode. The electrochemical activities of RPTP graphene modified electrode were characterized with cyclic voltammetry and chronoamperometric measurements thus allowing us to study the biocatalysis of reduction of H2O2. It is expected that the conjugated CS-GA linked to the molecules of RPTP improves the electron transfer between active center of enzyme and the graphene electrode. The analytical performance of the proposed sensor was evaluated for the determination of H2O2. The fabrication of the graphene modified electrode will provide a new sensor based on the highly stable royal palm tree peroxidase for sensing of H2O2 in real samples.
Results and discussion
Scanning electron microscopy characterization of the graphene modified electrodes
The morphologies of the surface of bare SPGE, CS SPGE and CS-GA-RPTP SPGE are displayed in Fig.1. From Fig.1A, it could be seen the graphene sheets on the electrode surface. As it is shown in Fig.1B, the crumble graphene sheets are densely covered by aggregates molecules of CS. After the CS-GA was mixed with peroxidase and immobilized on the surface of the electrode, it can be seen in Fig.1C that the CS-GA-RPTP aggregates are covering graphene sheets on the electrode surface.
Electrochemical characterization of CS-GA-RPTP graphene modified electrode
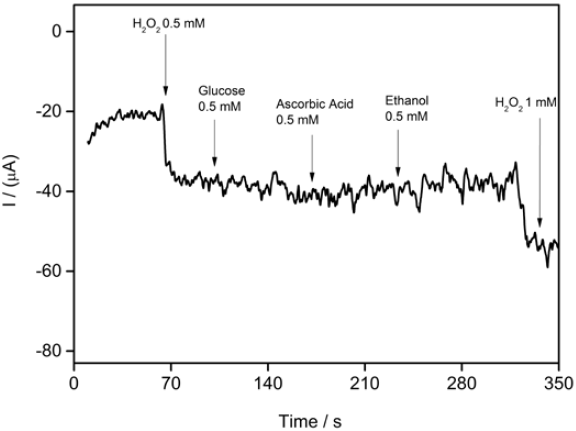
The electrochemical response of a biosensor to K3Fe(CN)6/ K4Fe(CN)6 by CV is established to evaluate the barrier created after each modification step. CVs of ferri/ferrocyanide couple using modified and unmodified SPGE are shown in Fig.2. It can be seen from the CV for bare graphene electrode a well pair of redox peaks at 256 mV and at -64 mV for anodic and cathodic peak, respectively. It can be observed an increase in both cathodic and anodic current when the conjugate CS-GA was deposited on the surface of SPGE. Thus the presence of CS-GA conjugate plays an important role in the electron transfer between redox species in solution and the graphene electrode surface probably due to the increasing of electroactive area of the transducer.
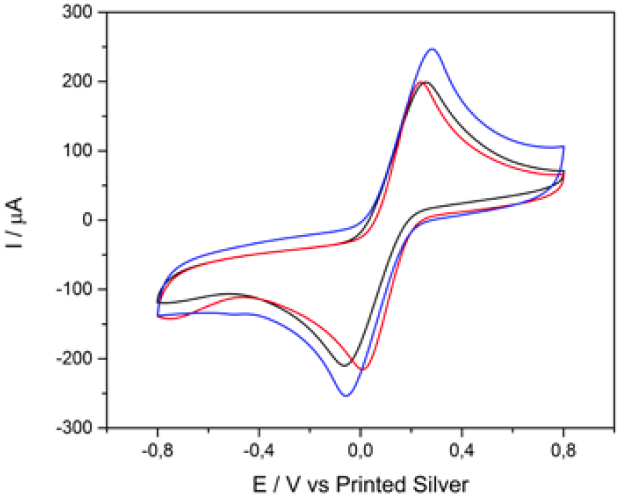
Fig. 2 Cyclic voltammograms of bare SPGE (black), CS SPGE (red) and CS-GA-RPTP SPGE (blue) in the presence of 10 mM K3Fe(CN)6 containing 0.1 M KCl, scan rate of 50 mV s-1.
Graphene is a 2D nanomaterial with special electrochemical properties and the material has received special attention since freestanding isolation in 2004 [1]. From the CVs in Fig.2, it is apparent that the combination of graphene with CS-GA shows an increase in the surface area of the transducer, which is reflected in the current values obtained with the CS-GA SPGE. The presence of GA as a crosslinker agent between enzyme-electrode combined with the porosity and the presence of different functional groups in the graphene structure improves the electron transfer between RPTP and the electrode surface. On the other hand anodic and cathodic currents of SPGE modified with CS in the absence of GA showed no significant improvements in electron transfer.
Electrocatalytic response of CS-GA-RPTP graphene modified electrode towards hydrogen peroxide
The CVs of CS-GA-RPTP SPGE in pH 7.0 PBS at 100 mV s-1 in the absence and in the presence of 0.5 mM of H2O2 are shown in Fig.3. As it is shown in Fig.3 there was no increase in the CV for CS-GA-RPTP graphene modified electrode in the absence of H2O2.
Fig. 3 Cyclic voltammograms of CS-GA-RPTP graphene modified electrode in the absence (blue) and in the presence (red) of 0.5 mM of H2O2, scan rate of 100 mV s-1.
Interestingly, when 5 mM H2O2 is added to the buffer solution, the voltammograms shows a high reduction current (cathodic peak at -1000 mV), which could be attributed to the electrocatalytic reduction of H2O2 by the presence of RPTP acting as an efficient biocatalyst. Most of biosensor based on plant peroxidases reported applied potential higher than the redox potential of our biosensor [11, 15]. At this lower work potential the number of interferences in real samples could be minimized (see Fig.6). As is presented in previous reports [10, 16-18], the electrocatalytic cycle of peroxidases in the reduction of H2O2 at peroxidase modified electrodes can be expressed as follows:
After interaction with H2O2, the heme group of peroxidase, RPTP-Fe(III) immobilized on the CS-GA SPGE was oxidized by H2O2 to form compound I (Eq. 1), which is a two-equivalent oxidized form containing oxyferryl heme group and the porphyrin p cation radical. Compound I has electrocatalytic activity; its porphyrin radical obtains one electrode from the electrode, then forming compound II. Compound II then is reduced back to RPTP native form by accepting one electron from the electrode as is shown in Eq. 3 [18]. These results suggest that the electrocatalytic reduction of H2O2 can be achieved by RPTP immobilized on the surface of a CS-GA SPGE.
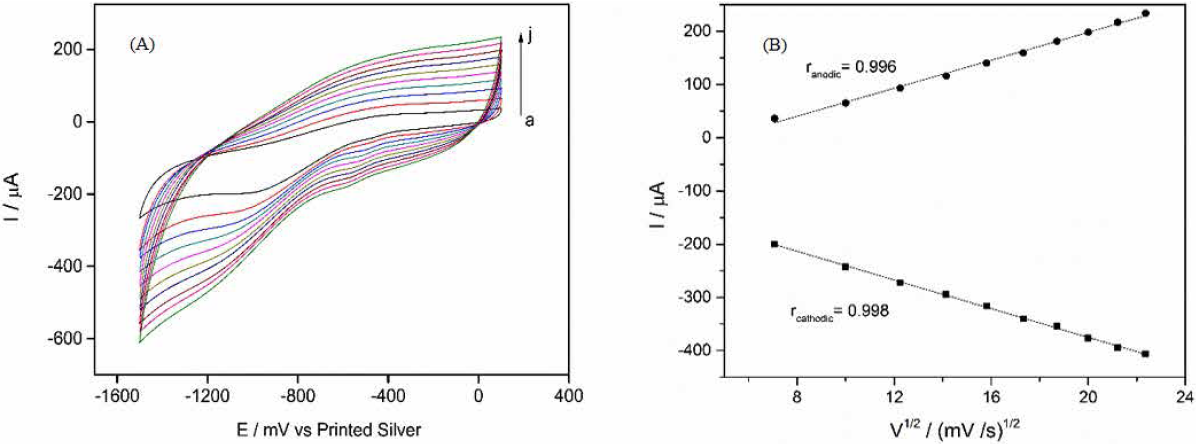
Effect of scan rate
Fig.4 presents the CVs of CS-GA-RPTP SPGE in 0.1 M pH 7.0 PBS solution at different scan rates. From the Fig.4 it can be observed that increases in scan rate produces diffusion of the electroactive species to the transducer surface (ranging from 100 to 500 mV s-1) and an increase in both cathodic and anodic current peaks is obtained. A linear increase in the square root of scan rate and anodic and cathodic peaks indicate a surface-controlled quasi reversible process. The linear regression equations were: I pc = -105.7 - 13.5v (r = 0.998) and I pa = -65.1 + 13.15v (r = 0.996), respectively.
Chronoamperometric measurements
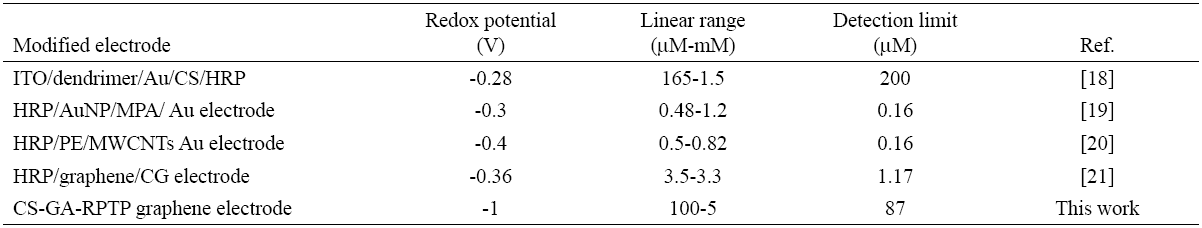
The amperometric response of H2O2 to CS-GA-RPTP graphene modified electrode was evaluated using chronoamperometric mode. Fig.5A displays a typical current-time plot for RPTP sensor on successive additions of 0.5 mM H2O2 to 7 mL pH 7.0 PBS solution at an applied potential of -1000 mV. The reduction current reached a stable value upon addition of an aliquot of H2O2 (Fig.5A) to the stirring buffer solution and the steady state current was achieved within 5 s, which strongly evidence a fast electron transfer process between redox center of the RPTP and the modified surface electrode. It is faster than the reported results of 8 s for HRP immobilized on a glassy carbon electrode modified with gold nanoparticles [16], and 20 s for a HRP adsorbed on a glassy carbon electrode modified with thionine [11]. The fast response exhibited by the RPTP sensor could be produced by the direct communication between the RPTP and the modified surface of the graphene electrode. Control experiments for chronoamperometric response of bare graphene electrode and SPGE-CS are presented in Fig.5C.
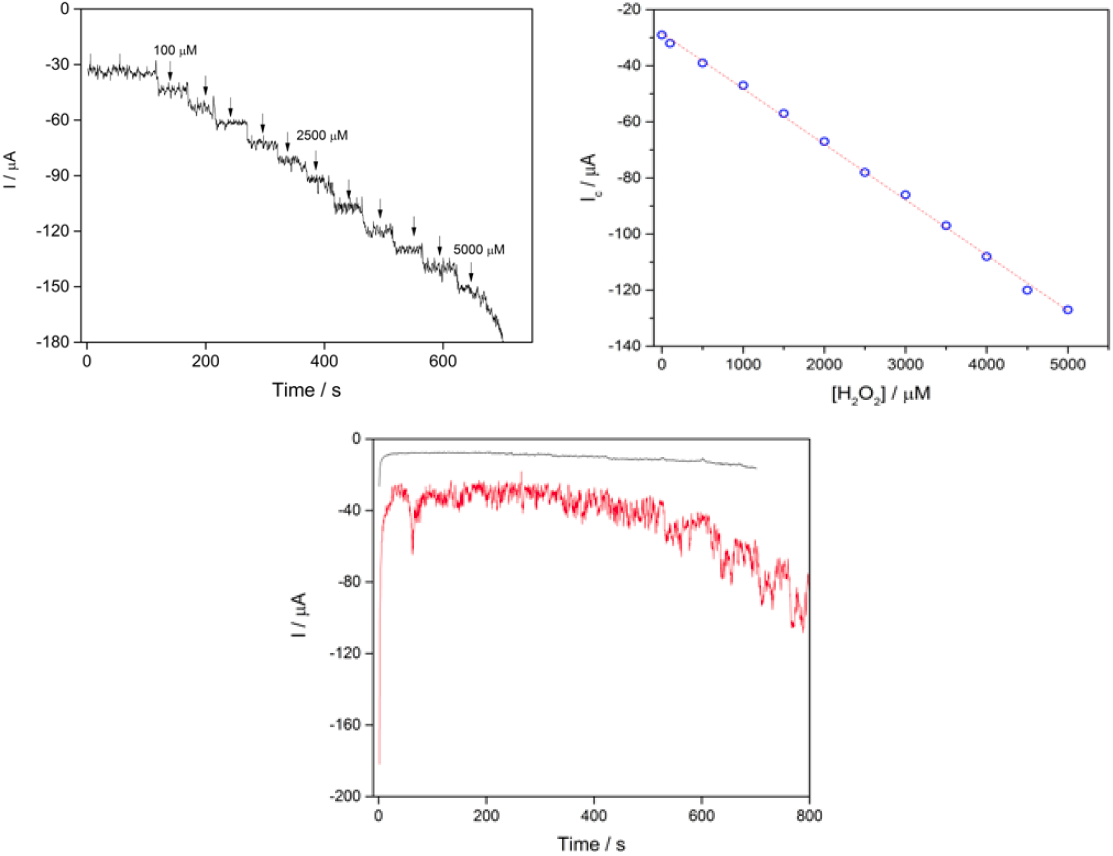
Fig. 5 (A) Chronoamperometric response of RPTP at the CS-GA graphene electrode in the presence of different amounts of H2O2 into 7 mL of PBS 10 mM pH 7.0 (vs silver printed); (B) calibration curve of the cathodic current vs concentration of H2O2; (C) control experiments for bare SPGE (-) and SPGE-CS (-) under successive additions of H2O2 (vs silver printed).
A linear response range of the sensor towards different H2O2 was from 100μM to 5 mM, and the linear equation regression was I = -28.4078 - 0.01979 [H2O2], with a correlation coefficient of 0.99851. The limit of detection was 87 μM (S/N = 3), and the linear range was in a wide range of 100 μM to 5 mM with a sensitivity of 0.019 μA/μM (Fig.5B). Table 1 compares the analytical performance obtained in previous reports and results obtained in this study.
The stability of CS-GA-RPTP SPGE was evaluated by 30 continuous cyclic scans. No obvious variations of the voltammetric waves was found (S1 supporting information). After storing 3 weeks at 4°C, the graphene modified electrode remained its bioelectrocatalytic activity, it was evidenced by the values in the amperometric signal close to the initial current response. This long-term stability could be attributed to the strong interactions between the graphene modified electrode surface and the amino and carboxylic groups of the RPTP.
Study of interferences
Fig. 6 shows the interference studies of the biosensor response after additions of 5mM of glucose, ascorbic acid and ethanol. The study showed no significant signal to the interferences molecules assayed thus indicating a high selectivity and specific response towards H2O2.
Experimental Section
Chemicals and materials
Peroxidase from Royal palm tree peroxidase (Roystonea regia) was purified from leaves of palm tree according to the procedure used by Sakharov et al. [14]. Buffer salts (KH2PO4 0.05M and Na2HPO4 0.05M), chitosan (0.025% w/v in acetic acid solution), glutaraldehyde (2% w/v) and H2O2 (30% w/w) solutions were of analytical grade and obtained from Sigma-Aldrich Company (USA). All aqueous solutions were prepared using water purified with a Milli-Q system (Millipore, Bedford, USA). Screen printed graphene electrodes (SPGE, 110GPH) were provided by DropSens (Oviedo, Spain). Each electrode consisted of a graphene working electrode (4 mm of diameter), a silver reference electrode, and a carbon counter electrode. SPGE modified with CS-GA/RPTP was fitted into a methacrylate electrochemical cell (Dropsens, Spain).
Apparatus and procedures
In order to characterize the modification of graphene electrodes, Scanning Electron Microscopy (SEM) analysis was performed using a Quanta FEG 650 SEM microscope (Oregon, USA) with a maximal spatial resolution of 1.0nm. An acceleration voltage of 5.0 kV and a working distance of 2.6 nm was used.
Cyclic voltammetry and chronoamperometric experiments were carried out with a Autolab PGSTAT101 (Echo Chemie, Utrecht, The Netherlands) controlled by NOVA 1.10.1.9 software (Metrohm, Filderstand, Germany) in a screen printed three electrode configuration. Screen printed graphene electrodes (SPGE, 110GPH) were provided by DropSens (Oviedo, Spain). Each electrode consisted of a graphene working electrode (4 mm of diameter), a silver reference electrode, and a carbon counter electrode. SPGE modified with CS-GA/RPTP was fitted into a methacrylate electrochemical cell (Dropsens, Spain). All the experiments were carried out at room temperature. Before each CV experiment the work solution was magnetic stirred for 30 s. For chronoamperometric measurements, the working potential was set at -1000 mV and the solution was stirred gently with a magnetic stirrer before and during addition of hydrogen peroxide solutions.
Preparation of graphene electrode modified with Royal palm tree peroxidase and chitosan-glutaraldehyde
A 0.25 mg mL-1 CS solution was prepared by sonication of 5 mg of CS into 2 mL acetic acid (2%wt solution). Then 10 μL of CS solution were mixed with a RPTP solution and dispersed ultrasonically for 10 min. The enzyme-CS solution was mixed homogeneously with 10 μL of 2% GA (water solution) and dispersed ultrasonically for another 10 min. Finally 10 mL of CS-GA-RPTP solution was dropped on the surface of graphene electrode and it was dried for 3 h at room temperature. The surface of electrode was gently rinsed with buffer PBS pH 7.0 to remove unbounded CS, GA and RPTP molecules. The graphene modified electrode was stored at 4°C awaiting for electrochemical measurements. Three modified graphene electrodes were similar prepared for each set of measurements.
Interference studies
Detection of potential interferences such as glucose, ethanol and ascorbic acid, were evaluated in concentrations of 0.5 mM under optimized experimental conditions. The changes in the current signal of 0.5 and 1 mM were compared in the absence and in the presence of selected interferences.
Conclusions
The presented study was designed to fabricate and evaluate a novel sensor for detecting H2O2 based on the immobilization of Royal palm tree peroxidase (Roystonea regia) in combination with chitosan and cross-linking of glutaraldehyde on screen-printed graphene electrode. Peroxidase from royal palm tree peroxidase immobilized on graphene surfaces has shown to be an alternative biocatalyst to the commercial available HRP. The resulting graphene modified electrode exhibited a fast direct electron transfer and a good performance towards H2O2 determination with a wider linearity range and a lower detection limits. The biosensor exhibited a negligible amperometric response towards glucose, ascorbic acid and ethanol. Further research in this novel biosensor will provide a new sensing alternative for the detection of H2O2 in real samples.











 nueva página del texto (beta)
nueva página del texto (beta)