Introduction
Juncus effusus L. (Juncaceae), a famous traditional Chinese medicine (TCM) herb, is often found in the wetlands and coastal marshes of South China and used as a sedative, anxiolytic, antipyretic, and detumescence agent [1]. The species is one of the traditional medicines recorded in the Chinese Pharmacopoeia and the medullae can be used in TCM for treatment of various diseases, such as fidgetiness and insomnia, which are anxiety-like psychiatric symptoms [2], and the traditional use of which in China and Japan as antipyretic and antiphlogistic [3], and anticancer agents [4]. Previous investigation of secondary metabolites from J. effuses resulted in the isolation of phenanthrene derivatives [5-7], benzocoumarins [8], triterpenes [9, 10], flavonoids [11], phenolic acid derivatives [12], and steroids [13, 14]. As a part of our continuous study aiming at the discovery of new bioactive compounds from J. effuses [15], this work reports the isolation and characterization of a new sesquilignan effususin E (1) and a new long chain fatty enamide effususin F (2), together with eight known compounds 2-O-p-coumaroyl glyceride (3) [12], 2-O-feruloyl glyceride (4) [16], balanophonin B (5) [17], β-sitosterol (6), stigmast-4-en-6β-ol-3-one (7) [18], 3β-hydroxy-5α,8α-epidiocyergosta-6E, 22E-dienne (8) [19], 5-hydroxymethyl-2-furancarboxaldehyde (9) [20], and 3-oxo-α-ionol (10) [21] (Fig. 1). The cytotoxic activities of the two new compounds were evaluated against SHSY-5Y, SMMC-7721, HepG-2, Hela, and MCF-7 cancer cell lines using the CCK-8 method [22]. In addition, the new isolates were evaluated for their inhibitory effects on NO production in lipopolysaccharide-activated murine macrophage RAW264.7 cells [23, 24].
Results and Discussion
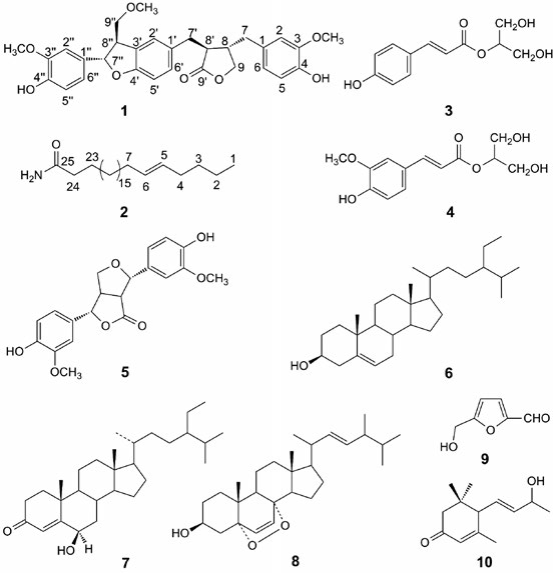
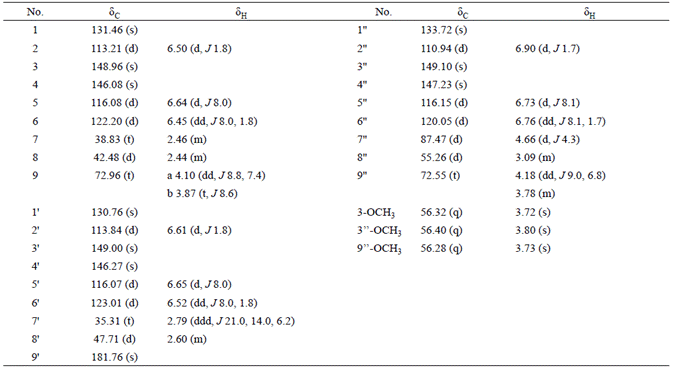
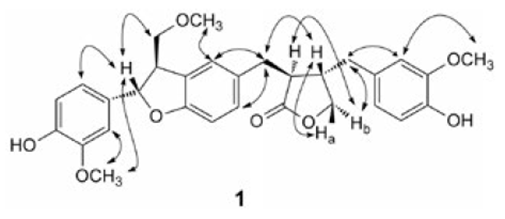
Effususin E (1) was isolated as yellow gum with the formula as C30H32O8 determined by HREIMS at m/z 520.2072 [M]+ (calcd 520.2097 for C30H32O8). The 1H NMR (Table 1) spectrum showed three singlets δH 3.72s, 3.73s, and 3.80s assignable to three methoxy groups. Three ABX systems were exhibited at δH 6.50 (d, J 1.8, H-2), 6.64 (d, J 8.0, H-5), 6.45 (dd, J 8.0, 1.8, H-6), 6.61 (d, J 1.8, H-2'), 6.65 (d, J 8.0, H-5'), 6.52 (dd, J 8.0, 1.8, H-6'), 6.90 (d, J 1.7, H-2''), 6.73 (d, J 8.1, H-5''), and 6.76 (dd, J 8.1, 1.7, H-6''). In the aliphatic region, the proton signals of four -CH and four -CH2 groups appeared as two spin systems corresponding to -CH-CH-CH2and -CH2-CHCH-(CH2)2-, which were revealed by the 1H-1H COSY and HMBC experiments (Fig. 2). In addition, the 13C NMR (Table 1) spectrum showed the characteristic signals due to a benzofuran unit resonating at δC 87.47d (C-7''), and 55.26d (C-8''), and a furanone ring resonating at δC 42.48d (C-8), 72.96t (C-9), 47.71d (C-8'), and 181.76s (C-9'). Therefore compound 1 was sesquilignan. The assigned structure was further confirmed by analysis of HMBC and 1H-1H COSY correlations (Fig. 2). Furthermore, the correlations between H-7'' and H-9'', Hb-9 and H-8', Hb-9 and H-7, H-7' and H-8, Ha-9 and H-8, and H-2'' and H-OCH3 (3.80q), H-2' and H-OCH3 (3.73q), H-2 and H-OCH3 (3.72q) in the NOESY spectrum determined the configuration of 1 and the station of -OCH3 (Fig. 3). The relative configuration of 1 was also assigned by comparison of NMR data and optical rotation with those previously described for compound 10 [25], the optical rotation for which was same as that of 1, suggesting the same configuration. On the basis of these evidences, the structure of compound 1 was established as (3S,4S)-4-(4hydroxy-3-methoxybenzyl)-3-(((2S,3R)-2-(4-hydroxy-3-methoxyphenyl)-3-(methoxymethyl)-2,3-dihydrobenzofuran-5-yl) methyl)dihydrofuran-2(3H)-one, named effususin E in Fig. 1.
Effususin F (2) was obtained as white powder. The molecular formula C25H49ON was determined by HREIMS at m/z 379.3821 [M]+ (calcd 379.3814 for C25H49NO) from the molecular ion peak observed in the EIMS and HREIMS measurement. The IR spectrum showed double absorption bands for -NH2 group at 3396 and 3188 cm-1, and a broad overlap absorption band of a carbonyl group and a double bond at 1647 cm-1. Its 1H NMR showed one methyl at δH 0.88 (t, J 5.9 Hz, H-1), four methylenes at δH 2.00 (m, H-4 and H-7), δH 1.62 (m, H-23), δH 2.22 (t, J 7.6 Hz, H-24), a double bond at δH 5.33 (dt, J 15.5, 4.8 Hz, H-5), δH 5.36 (dt, J 15.5, 4.7 Hz, H-6), and two protons of an NH2 group at δH 5.75 (s) and 5.49 (s), which is characteristic of the unsaturated long chain fatty amide derivative. The 13C NMR and HREIMS showed that compound 2 has total 25 carbon atoms, and they are all located at the long chain moiety. In HMBC spectrum of 2, appearance of key correlations between δH 2.00 (m, H-4 and H-7) and δC 130.03 d (C-5), δC 130.00 d (C-6) suggested the double bond was located at C-5 and C-6, which was further confirmed by the fragment ion peak at m/z 336 [M C3H7]+ in the EIMS of 2 caused by Mclafferty rearrangement. The E configuration for the double bond was suggested by its coupling constant (J 15.5 Hz). The HMBC also showed the correlations of δH 0.88 (t, J 5.9 Hz, H-1) and δc 22.82t (C-2), 32.04t (C-3), δH 1.62 (m, H-23) and δc 175.97s (C-25), δH 2.22 (t, 7.6 Hz, H-24) and δc 25.67t (C-23), 175.97s (C-25) (Fig. 2). Thus, the structure of 2 was elucidated as (E)-pentacos-20-enamide, named effususin F.
Compounds 1 and 2 were evaluated for their in vitro cytotoxicities against five human cancer cell lines (SHSY-5Y, SMMC-7721, HepG-2, Hela, and MCF-7) by using CCK-8 assay with paclitaxel as the positive control (Table 2), but only 1 exhibited weak cytotoxicity against SMMC-7721 cell line with IC50 value of 57.5 μm. Inhibitory effects of 1 and 2 on NO production in lipopolysaccharide-induced RAW264.7 macrophages were also evaluated, and they were found to exhibit good inhibitory activities with IC50 values of 8.59 and 13.73 μm, respectively (Table 2).
Table 2 Cytotoxicity and Anti-inflammation Effect of Compounds 1 and 2 (IC50 ± S.D. [μM]).
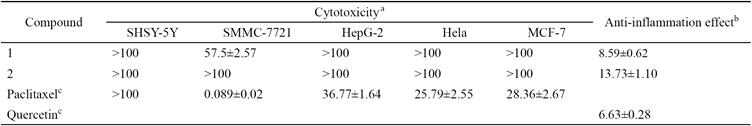
a The Cytotoxicity of the compounds was evaluated by CCK-8 assay against five human cancer cell lines.
b The anti-inflammatory effects on nitric oxide production in lipopolysaccharide activated murine macrophage RAW264.7 cells.
c Paclitaxel was used as positive controls in Cytotoxicity assay. Quercetin was used as positive control in Anti-inflammation assay.
Juncusol and dehydrojuncusol, the main phenanthrene in J. acutus, were already reported in 2007 to show strong anti-inflammatory activities by effectively reducing the iNOS protein expression in LPS-activated RAW 264.7 cells [3]. Subsequent publication by our group reported that sixteen phenanthrenes, the main constituents from J. effusus, displayed obvious anti-inflammatory effects by inhibition the NO production in LPS-activated murine macrophage RAW 264.7 cells [26]. The phenanthrenoids may be regarded as main anti-inflammatory constituents of the J. herbs on dysuria, pharyngitis, aphtha, and trauma infections. This study revealed two nonphenanthrenes, a new sesquilignan (1) and a new long chain fatty enamide (2), exhibited good anti-inflammatory activities. From the above observations, both phenanthrenes and nonphenanthrenes seem to be all important for the anti-inflammatory of the J. effusus herbs. The anti-inflammatory study provided new class of natural anti-inflammatory agents.
Conclusions
A new sesquilignan effususin E (1) and a new long chain fatty enamide effususin F (2), together with eight known compounds were isolated from the medullae of J. effusus. Compounds 4, 5, 9, and 10 were reported from this genus for the first time. The cytotoxic activity of two new isolates 1 and 2 was evaluated against SHSY-5Y, SMMC-7721, HepG-2, Hela, and MCF-7 cancer cell lines, only 1 exhibited weak activity against SMMC7721 with IC50 value of 57.5 μm. Compounds 1 and 2 exhibited good inhibition of NO induced by lipopolysaccharide with IC50 values of 8.59 and 13.73 μm, respectively. The results suggested that 1 and 2 were also the active compounds as phenanthrenoid compounds in J. effuses medullae on pyretic and phlogistic diseases [5-7]. This study further supported the traditional use of which in China and Japan as antipyretic and antiphlogistic [3].
Experimental
General experimental procedures
UV spectra were obtained using a Shimadzu UV-2401PC spectrophotometer. A Bruker Tensor 27 FT-IR spectrometer was used for scanning infrared (IR) spectroscopy with KBr pellets. 1D and 2D nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AM-400 and Avance III-600 spectrometer with tetramethylsilane (TMS) as internal standard. Chemical shifts (δ) are expressed in ppm with reference to the solvent signals. EIMS and HREIMS were performed on a Waters Autospec Premier P776 spectrometer. Optical rotations were obtained using a Jasco P-1020 polarimeter. Column chromatography (CC) was carried out on Sephadex LH-20 gel (25-100 μm, Pharmacia Fine Chemical Co. Ltd.), RP-8 MB (100-40/75 μm, Fuji Silysia Chemical Co. Ltd.), and silica gel (200-300 mesh, Qingdao Haiyang Chemical Co. Ltd., Qingdao, P. R. China). Prep. TLC (PTLC) was precoated with silica-gel GF254 (Qingdao Haiyang Chemical Co. Ltd., Qingdao, P. R. China). Thin layer chromatography (TLC) was carried out on silica gel G precoated plates (Qingdao Haiyang Chemical Co. Ltd., Qingdao, P. R. China), and spots were detected by spraying with 5% H2SO4 in EtOH followed by heating.
Plant material
The medullae of J. effuses was purchased in October 2012 in Bozhou, in the Anhui province of China. The species was identified by Prof. Kai-Jin Wang from the School of Life Sciences, Anhui University. A voucher specimen (No. 20121001) has been deposited in our laboratory.
Extraction and isolation
The air-dried medullae of J. effuses (6 kg) were powdered and extracted with 95% ethanol (3 × 70 L) at room temperature. The extracts were combined and concentrated under vacuum to give a residue (180 g). The residue was subjected to column chromatography over a silica gel (200-300 mesh), eluted with petroleum ether-acetone (from 10:1 to 1:1) to generate fractions 1-5. Fraction 2 (2.8 g) was subjected to medium pressure silica gel column chromatography eluted with petroleum ether-ethyl ether-acetone (20:2:1) to yield three fractions 2-1 to 2-3. Fraction 2-2 (1.21 g) was subjected to medium pressure silica gel column chromatography eluted with petroleum ether-acetone (30:1) to afford six fractions 2-2-1 to 2-2-6. Fraction 2-2-2 (860 mg) was subjected to silica gel column chromatography eluted with petroleum ether-acetone (40:1) to yield five fractions 2-2-2-1 to 2-2-2-5. Fraction 2-2-2-5 (600 mg) was chromatographed on Sephadex LH-20 (petroleum ether-CHCl3 -MeOH, 10:10:1) to afford compound 6 (300 mg). Fraction 3 (14.0 g) was subjected to chromatography over a silica gel (200-300 mesh) (petroleum ether-EtOAc, 5:1), and then Sephadex LH-20 (EtOH) to give fractions 3-1 to 3-3. Fraction 3-2 (412 mg) was chromatographed on Sephadex LH-20 (EtOH) to afford four fractions 3-2-1 to 3-2-4. Fraction 3-2-2 (260 mg) was chromatographed on Sephadex LH-20 (petroleum ether-CHCl3-MeOH, 5:5:1) to give four fractions 3-2-2-1 to 3-2-2-4. Fraction 3-2-2-2 (180 mg) was subjected to silica gel column chromatography eluted with petroleum etherethyl acetate (10:1) to afford 8 (80 mg) and the rest of mixture by preparation of PTLC to obtain compound 7 (25 mg). Fraction 3-3 (75 mg) was repeated chromatographed on Sephadex LH-20 (EtOH) to afford compound 10 (10 mg). Fraction 4 (11 g) was subjected to column chromatography over a silica gel (200-300 mesh), eluted with petroleum ether-EtOAc (5:1) to give fractions 4-1 to 4-5. Fraction 4-4 (300 mg) was repeated chromatographed on Sephadex LH-20 (EtOH) to obtain compound 2 (150 mg). Fraction 5 (20.5 g) was subjected to column chromatography over a silica gel (200-300 mesh), eluted with ether-EtOAc (4:1) to generate fractions 5-1 to 5-4. Fraction 5-2 (3 g) was applied to Sephadex LH-20 eluted with EtOH-H2O (from 20% to 100%) to generate fractions 5-2-1 to 5-2-3. Fraction 5-2-2 (1.2 g) was subjected to silica gel column chromatography (CHCl3) to afford fractions 5-2-2-1 to 5-2-2-6. Fraction 5-2-2-3 (230 mg) was applied to Sephadex LH-20 (EtOH-H2O, 40%) to obtain fractions 5-2-2-3-3 purified over Sephadex LH-20 (EtOH-H2O, 40%) to afford compound 1 (10 mg) and fractions 5-2-2-3-4 purified by silica gel column chromatography (CHCl3) to afford compound 5 (135 mg). Fraction 5-3 (2.8 g) was subjected to medium pressure silica gel column chromatography eluted with petroleum ether-acetone (5:1) to yield five fractions 5-3-1 to 5-3-5. Fraction 5-3-3 (115 mg) was chromatographed on Sephadex LH-20 (petroleum ether-CHCl3-MeOH, 5:1:1) and then by preparation of silica gel column chromatography (petroleum ether-EtOAc, 5:2) to afford compound 9 (20 mg). Fraction 5-3-4 (750 mg) was applied to Sephadex LH-20 (EtOH) to generate fractions 5-3-4-1 to 5-3-4-4. Fraction 5-3-4-2 (120 mg) was chromatographed on RP-8 (EtOH-H2O, 20%-80%) and then by preparation of silica gel column chromatography (CHCl3-MeOH, 25:1) to obtain compounds 3 (50 mg) and 4 (35 mg).
Effususin E (1)
Yellow gum; [α]24D + 24.9 (c 1.95, MeOH); UV(MeOH) λ max (log ε) 282 (3.71), 226 (4.11), 203 (4.72) nm; IR (KBr) v max 3441, 3432, 2957, 2925, 2854, 1759, 1631, 1517, 1463, 1452, 1384, 1275, 1238, 1206, 1156, 1124, 1033, 578, 563 cm-1; 1H and 13C NMR data (600 and 150 MHz, CD3OD), see Table 1; ESIMS m/z 519 (100) [M-H]-; HREIMS m/z, calcd. for C30H32O8 [M]+: 520.2097, found: 520.2072.
Effususin F (2)
White powder; [α]24D -11.0 (c 0.21, MeOH); UV(MeOH) λmax (log ε) 201 (3.46) nm; IR (KBr) vmax 3396, 3188, 3011, 2955, 2921, 2849, 1647, 1469, 1419, 1378, 1343, 1325, 1301, 1273, 1246, 1216, 1119, 722, 647 cm-1; 1H NMR (CDCl3, 400 MHz) δ 0.88 (3H, t, J1,2 = 5.9 Hz, H-1), 1.20-1.38 (34H, m, H-2, 3, 8-22), 2.00 (4H, m, H-4, 7), 5.33 (1H, dt, J5,6 = 15.5, J4,5 = 4.8 Hz, H-5), 5.36 (1H, dt, J5,6 = 15.5, J6,7 = 4.7 Hz, H-6), 1.62 (2H, m, H-23), 2.22 (2H, t, J23,24 = 7.6 Hz, H-24), 5.49 (1H, s, Ha-N) and 5.75 (1H, s, Hb-N); 13C NMR (CDCl3, 100 MHz) δ 14.27q (C-1), 22.82t (C-2), 32.04t (C-3), 27.33t (C-4, C-7), 130.03d (C-5), 130.00d (C-6), 29.37-29.90t (C-8-C-22), 25.67t (C-23), 36.10t (C-24), 175.97s (C-25); HREIMS m/z, calcd. for C25H49NO [M]+: 379.3814, found: 379.3821.
Cytotoxicity assay
The effects of compounds on cell proliferation and the viability of the SHSY-5Y, SMMC-7721, HepG-2, Hela, and MCF-7 cells were measured by using Cell Counting Kit-8 (CCK-8, Best Bio China) assay. Cells were seeded in 96-well plates at 5.0 × 103 cells/well in a final volume of 100 μl and treated with different concentrations (6.25, 12.5, 25, 50, 100 μM) of compounds or without tested compounds at given concentrations. After treatment for 48 h, CCK-8 (10 μl) was added to each well containing 100 μl culture medium, and the plate was incubated for 4 h at 37 °C. The absorbance of each well was measured at 450 nm with a microplate reader. The experiments were performed in triplicate, and the data are expressed as the means ± SD of three independent experiments.
Anti-inflammatory assay
The cytotoxicity of the tested compounds was evaluated by MTT (Sigma, USA) assays. RAW264.7 cells at 5 × 103 per well were seeded in 96-well plates. After 12 h incubation, the cells were treated with or without tested compounds for 24 h. Then, 20 μl MTT solutions (5 mg ml-1) were added to each well for another 4 h and the resulting crystals were dissolved in dimethyl sulfoxide (DMSO). The optical density was measured at 490 nm. The cytotoxicity was calculated from the plotted results using untreated cells at 100%.
The nitrite concentration was measured in supernatants of cultured RAW264.7 cells according to the Griess reaction as an indicator of NO production using an NO assay kit (Beyotime Insititute of Biotechnolgy, China). Cells were seeded in 96-well culture plates for 12 h incubation, and then pretreated with tested compounds (3 to 50 μM) and LPS (Sigma, USA, 1 μg mL-1 in PBS) for 24 h. 50 μl of cell-free supernatant was mixed with 100 μl of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride, and 2.5% phosphoric acid) and incubated at room temperature for 5 min. The concentration of nitrite was measured at 540 nm. Sodium nitrite (NaNO2) was used as a standard curve. The experiments were performed in triplicate, and the data are expressed as the means ± SD of three independent experiments.











 text new page (beta)
text new page (beta)