Introduction
The synthesis of hybrid inorganic-organic systems for human health has received considerable attention. The interest focuses on the synergistic properties, effects and new potential uses. In particular, hybrid materials prepared by intercalation of bioactive molecules into the galleries of layered double hydroxides (LDHs) result in a wide spectrum of versatile materials [1, 2]. The application field of these composites depends on the nature of the intercalated anion, thus different LDHs have been used as guests for nonsteroidal anti-inflammatory drugs [3, 4], sunscreen agents [5-7], molecules with antioxidant properties [8,9], anticancer drugs [10-12], antibiotics [13-16], and even for DNA and genes [17-19]. In this sense, many efforts have been made aiming to protect the bioactive molecules from degradation, or to enhance the bioavailability and release properties.
Layered double hydroxides belong to a family of interesting inorganic matrices, represented by the general formula
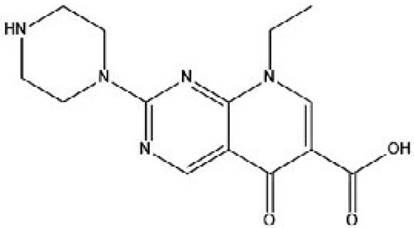
Pipemidic acid (PA), 8-Ethyl-5-oxo-2-piperazin-1-yl-5,8dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid (Scheme 1), is a derivative of piromidic acid. It is active against gram-negative bacteria as well as some gram-positive bacteria, but it is active against resistant bacteria to piromidic acid and nalidixic acid.
This work deals with synthesis of a hybrid material through the intercalation of pipemidic acid anions into galleries of MgFeAl LDH. The aim was to study their properties as antibacterial agent against E. coli and S. typhi strains.
Experimental
Sample Preparation
The pristine MgFeAl-CO3, containing carbonate ions in the interlayer region, was synthetized by conventional coprecipitation method under high supersaturation conditions [22]. A 1M Mg2+, Al3+, and Fe3+ solution was prepared by dissolving the corresponding nitrates salts in deionized water. The following molar ratios were used, Mg2+/(Fe3+ + Al3+) = 3, Al3+/Fe3+ = 3.6. Separately, a 1M aqueous solution containing Na2CO3 and KOH was prepared. The alkaline solution was added dropwise under vigorous stirring to the metallic solution until pH = 9 was reached. The resulting suspension was then aged at 80 °C for 16 h. At the end of aging, the solid was centrifuged and washed several times with deionized water. Finally, the solid was dried overnight at 120 °C in an oven yielding the sample named MgFeAl-CO3.
The MgFeAl-Cl LDH was prepared by suspending the MgFeAl-CO3 sample in a 0.1 M solution of NaCl. The above suspension was then titrated with HCl 0.1 M at pH = 5. The resulting solid was centrifuged and washed several times with CO2-free deionized water. The dried solid was stored under vacuum before using.
The intercalated MgFeAl-PIP sample (PIP are pipemidic acid anions) was synthetized by an anion exchange reaction [23]. Pipemidic acid is nearly insoluble in water, thus, to generate a soluble sodium salt, a suspension of pipemidic acid (2.8 mmol in 25 mL of CO2-free deionized water) was reacted with the stoichiometric amount of NaOH and the final pH was adjusted to 9. The resulting solution was bubbled with argon before adding MgFeAl-Cl LDH (molar ratio PIP/M3+ = 2). The bubbling was maintained for 1 h. Then, the system was sealed under argon. After seven days of stirring, the MgFeAl-PIP was then separated by centrifugation, washed several times with warm CO2-free deionized water, dried at 80 °C overnight and finally stored under vacuum.
Antibacterial activity
The Escherichia coli ATCC® 14028 and Salmonella typhi ATCC® 25922 strains were supplied from the Laboratorio Estatal de Salud Pública de Michoacán. The antibacterial activity of the pristine MgFeAl-Cl LDH and the MgFeAl-PIP composite, against both pathogenic bacteria, was evaluated by agar well diffusion method, according to the procedure previously reported [24-26]. The Minimum Inhibitory Concentration (MIC), defined as the lowest concentration of a particular antibiotic where the absence of visible growth is recorded, was also determined on plates of agar dilution and broth dilution assays. Similar techniques were used to determine the Minimum Bactericidal Concentration (MBC). The MBC is defined as the lowest concentration of an antibacterial agent needed to kill a particular bacterium. Both, agar dilution and broth dilution assays provided similar MIC and MBC values. Both cultures were grown and maintained in tripticaseine broth medium. A starter culture of each strain was inoculated with fresh colonies and then incubated for 24 h in tripticaseine medium, the inoculum consisted of 109 bacteria/mL. Fresh medium was inoculated in tests tubes with the starter culture and grown at 35.5 °C under continuous stirring at 30 rpm. MgFeAl-Cl or MgFeAl-PIP were added to bacterial cultures at an average MBC, previously determined for each material. Then, samples were taken at different intervals of time and seeded in Petri dishes previously loaded with 30 mL of selective agar. Finally, the dishes were incubated at 35.5 °C under aerobic conditions and the number of surviving colonies was determined. As a control, a plate was inoculated without bactericide material. All the materials were sterilized during experiments with bacteria.
Characterization
Powder X-ray diffraction (PXRD) patterns were recorded using CuKα1 (λ = 1.5406 Å) on a Philips X'Pert instrument operating at 45 kV and 45 mA in the 2θ range of 4-80°.
Infrared spectra were recorded in the range of 4000400 cm-1 using an FTIR Nicolet Magna IR 750 infrared spectrometer. Samples were diluted with KBr and then pressed to form discs.
Results and discussion
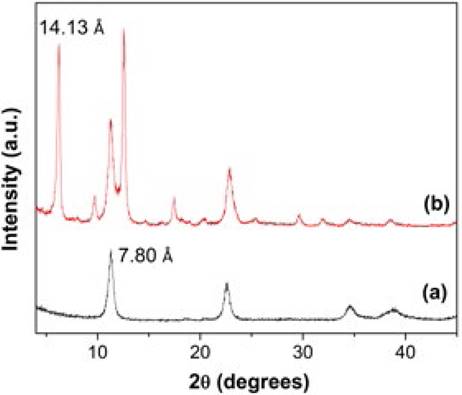
PXRD patterns of MgFeAl-Cl LDH and MgFeAl-PIP composite are given in Fig. 1 and the unit cell c parameters are shown in Table 1.
Table 1 The unit cell c parameters obtained for pristine MgFeAl-Cl LDH and MgFeAl-PIP composite.
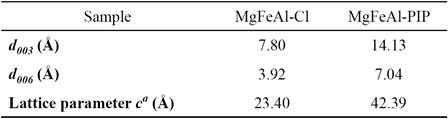
a The unit cell c parameter was calculated according to c = 3d 003.
The XRD reflections of the pristine MgFeAl-Cl LDH are attributed to hydrotalcite structure (JCPDS 22-700) and confirm the formation of a layered structure with a basal spacing of 7.80 Å. This value agrees well with those reported for MgFeAl-Cl LDH with similar composition [27, 28]. Thus, the sharp peak at 2θ = 11.33° corresponds to the (003) plane of the hexagonal structure with rhombohedral symmetry. The position of the (003) reflection depends on the number, orientation and nature of anions hosted in the interlayer region. The (003) peak for the MgFeAl-PIP composite is observed at 2θ = 6.25° (14.13 Ǻ). The increase of the interlayer distance from 7.80 to 14.13 Ǻ indicates the effective intercalation of the PIP anions. Thus, as a result of the increase in the basal spacing after exchange, the lattice structure of LDH expanded to the c-axis direction. As the brucite-like layer thickness is 4.8 Ǻ [29], the gallery height can be estimated to be 9.33 Ǻ for MgFeAl-PIP. This gallery height seems to be small to allow hosting such a big anion molecule. Such dimension can be only explained assuming that the intercalated anions molecules are arranged in a monolayer with a tilted orientation toward the brucite-like sheets. Nevertheless, the peaks observed near to 2θ = 11.33°, and 22.89° (Fig. 1b) indicate that the exchange of chloride anions by pipemidic acid anions was incomplete. Additionally, the small peak at 2θ = 9.75° might be due to intercalation of some pipemidic acid anions with the main axis oriented parallel to the layers of the LDH. It is worth mentioning that none reflection attributable to the pipemidic acid sodium salt was observed. In fact, XRD analysis of the pipemidic acid sodium salt (not included) showed the presence of a completely amorphous phase. However, as Fig. 1b indicates, XRD pattern of the MgFeAl-PIP hybrid material is not a combination of the XRD patterns of the pristine MgFeAl-Cl LDH and the pipemidic acid sodium salt, which indicates that, indeed, the intercalation of the pipemidic acid anions was successful.
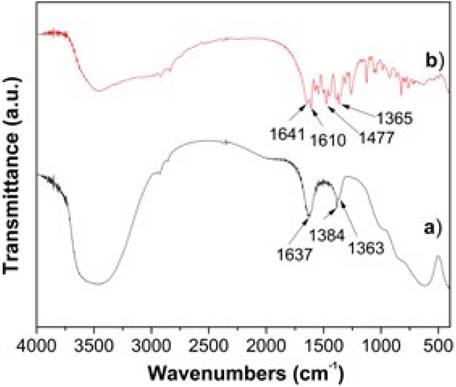
The FTIR spectra for MgFeAl-Cl LDH and MgFeAl-PIP composite are shown in Fig. 2. It is known that pipemidc acid do not show a ʋ(C=O) absorption because the carboxylic group is deprotonated and the molecule exists as a zwitterion [30, 31]. Instead, two characteristic bands in the range of 1650-1510 cm-1 and 1400-1280 cm-1 are typical for similar ionic carboxylates [32]. These bands are attributable to asymmetrical and symmetrical stretching vibrations of COO- group, respectively. The FTIR spectrum (not shown) of the pipemidic acid shows two strong bands at 1640 and 1618 cm-1 which are assigned to the carbonyl ring group and to the asymmetrical stretching vibrations of COO- group, respectively. The pipemidic acid sodium salt (for clarity not shown) shows a very similar FTIR spectrum to that of pipemidic acid, confirming that the band at 1640 cm-1 (1633 cm-1 for the sodium salt) belongs to the stretching vibrations of COO- group.
For both MgFeAl-Cl LDH (Fig. 2a) and MgFeAl-PIP composite (Fig. 2b), the broad absorption band around 3500 cm-1 is due to O-H stretching vibration of the hydroxyl groups conforming the brucite-like layers and water in the interlaminar region. The O-H bending vibration of the interlayer water is observed at 1637 cm-1. Besides, MgFeAl-Cl LDH exhibits two bands situated at 1384 and 1364 cm-1 which can be ascribed to ʋ3 modes of the carbonate ion.
This indicates that a scarce amount of
As a consequence of the antibiotic molecule intercalation, the MgFeAl-PIP composite shows a rather complex FTIR spectrum composed by a combination of IR bands belonging to the layered phase and to the antibiotic molecule. The band at 1641 cm-1 is ascribed to the carbonyl ring group, while the band at 1610 cm-1 belongs to the asymmetrical stretching vibrations of COO group.
Results from both, XRD and FTIR analysis, confirm the intercalation of the antibiotic molecule into the galleries of the MgFeAl-LDH.
The MIC and the MBC values are reported in Table 2. The results indicate that MgFeAl-PIP hybrid material is slightly more effective against E. coli than S. typhi. The MgFeAl-Cl and MgFeAl-NO3 (included as a reference) did not show antimicrobial activity. As a comparison, the MIC of pure pipemidic acid were found to be 1.56 (g/mL and 3.13 (g/mL against E. coli and S. typhi, respectively, suggesting that PA is, in fact, twice more active for E. coli than S. typhi [36].
Table 2 The MIC and the MBC values of layered and composite materials against E. coli (Gram negative) and S. typhi (Gram negative) bacteria.
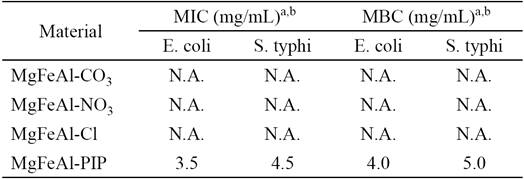
a N.A. No Activity, b on the basis of the mass of material.
In order to study the kinetics of the antimicrobial action of MgFeAl-LDHs (as reference) and MgFeAl-PIP composite a time-kill test at concentrations corresponding to the MBC value (Table 2) was achieved. As shown in (Fig. 3), E. coli bacteria multiply rapidly with time in presence of carbonate and nitrate containing MgFeAl-LDH, the number of colonies duplicated after 20 and 93 min, respectively, confirming null antimicrobial activity. As to MgFeAl-Cl LDH, it behaves almost as a bacteriostatic material during time. In this regard, it has been proven that other common LDHs such as ZnAl-CO3[37, 38], MgAl-CO3 and MgAl-NO3[39] do not exhibit any antibacterial activity against E. coli. Same conclusion was established for MgAlNO3 against S. aureus [40]. This demonstrates that LDHs do not exhibit any antibacterial characteristic by themselves, even if they contain chloride anions in their interlayer region. Finally, based on the results in Fig. 3, it is clear that the effect of the inorganic compensating anion (
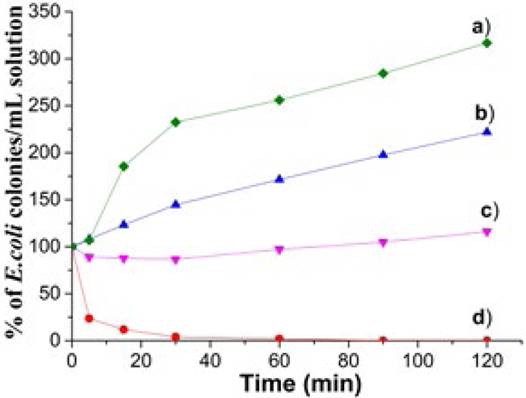
Fig. 3 Percentage of E. coli colonies surviving in culture media in presence of a) MgFeAl-CO3 LDH, b) MgFeAl-NO3 LDH c)MgFeAl-Cl LDH and d) MgFeAl-PIP hybrid material. Each material was evaluated at MBC.
On the other hand, Fig. 4 shows results of kinetics of antibacterial tests for MgFeAl-LDHs and MgFeAl-PIP composite against S. typhi. As observed for E. coli in Fig. 3, none of the inorganic anion containing LDHs showed antibacterial action.
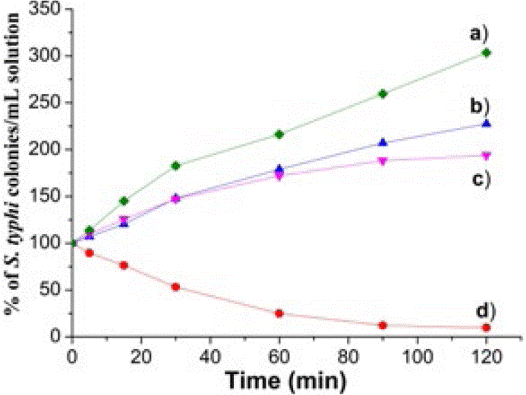
Fig. 4: Percentage of S. typhi colonies surviving in culture media in presence of a) MgFeAl-CO3 LDH, b) MgFeAl-NO3 LDH c) MgFeAl-Cl LDH and d) MgFeAl-PIP hybrid material. Each material was evaluated at MBC.
In this case, MgFeAl-Cl allows rapid development of colonies of S. typhi. Conversely, MgFeAl-PIP hybrid material showed good activity to eliminate bacteria, as only 12% of colonies survived after 90 min of exposure. As previously pointed out by results of MIC and MBC values, MgFeAl-PIP is less active to eliminate S. typhi than E. coli pathogens. These results are in good agreement with the data previously reported for the antibacterial activity of supported gold nanoparticles against E. coli and S. typhi [24]. Independently of the bactericide material, it is harder to kill S. typhi than E. coli because the first one has a very complex and resistant plasmatic membrane [41].
Conclusions
MgFeAl-PIP composite was prepared by exchanging pipemidic acid anions into the interlayer space of MgFeAl-Cl LDH. The exchange degree was not complete, as some Clanions remained in the interlayer space. The gallery height estimated to be 9.33 Ǻ suggested that pipemidic acid anions were intercalated as a monolayer in a tilted mode. However, some molecules were probably intercalated parallel to brucite-like sheets. MgFeAl-PIP hybrid showed efficient antibacterial activity against E. coli and S. typhi. The pristine MgFeAl LDH chosen in this work resulted to be a very good biocompatible inorganic host, as it cares not killing bacteria by itself, but it allows driving the release of biologically active molecules into the media growth with time. MgFeAl-PIP composite eliminated all colonies of E. coli after 90 min of exposure and showed good activity to eliminate S. typhi bacteria at times as short as 120 min.











 nova página do texto(beta)
nova página do texto(beta)