Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.59 n.4 Ciudad de México Oct./Dec. 2015
Article
Characterization of Thin Films Deposited by Physical Vapor Deposition (PVD), Using Electrochemical Impedance Spectroscopy (EIS) Technique
Jorge Morales Hernández,*1 Araceli Mandujano Ruíz,1 Julieta Torrez-González,1 René Antaño López,1 Federico Castañeda Zaldivar1 and Francisco Javier Espinoza Beltrán2
1 Centro de Investigación y Desarrollo Tecnológico en Electroquímica S.C., Parque Tecnológico Querétaro S/N, Sanfandila, Pedro Escobedo, Querétaro. C.P. 76703, México. Tel. +52 (442) 211-6035. jmorales@cideteq.mx*; amandujano@cideteq.mx; jtorres@cideteq.mx; rantano@cideteq.mx; fcastaneda@cideteq.mx
2 Centro de Investigación de Estudios Avanzados del I.P.N., Libramiento Norponiente 2000, Real De Juriquilla, 76230 Santiago de Querétaro, México. Tel. (442) 2119913. fespinoza@qro.cinvestav.mx
Received November 27th, 2015;
Accepted January 29th, 2016.
Abstract
In this paper the performance against corrosion of a thin film of NiCu deposited by Physical Vapor Deposition at two different times of deposition, was evaluated. Electrochemical Impedance results in NaCl solution, showed a gradual increase in resistivity due to the degradation of the film (delamination) for both times, along with the deposition of corrosion products on the coating generated by recombination of substrate oxides.
Key words: Corrosion; DC sputtering; Electrochemical Impedance Spectroscopy (EIS); nanodefects; PVD; Thin films.
Resumen
En el presente trabajo se evaluó el desempeño ante la corrosión de una película delgada de NiCu depositada por la técnica de Deposición de vapor física a dos diferentes tiempos de deposición. Los resultados de Espectroscopía de Impedancia Electroquímica en disolución de NaCl mostraron un aumento gradual de la resistividad como consecuencia de la degradación de la película (delaminación) para los dos tiempos, junto con la deposición de productos de corrosión generados sobre el recubrimiento por la recombinación de los óxidos del sustrato.
Palabras Clave: Corrosión; DC sputtering; Espectroscopía de Impedancia Electroquímica; Nanodefectos; Películas delgadas; PVD.
1. Introduction
Physical vapor deposition (PVD) technology is used to deposit thin films and coatings in the solid state for various industrial sectors. The wide variety of applications is mainly decorative although it is useful for development of engineering processes in chemical, nuclear, microelectronics, manufacturing and related industries. Their use has been increased together with the development of new materials and the combination of technology demands such as biocompatibility, high temperature strength, impact strength, wear and corrosion resistance, etc. [1]. Thin films development by PVD have reported coatings with low friction coefficient, high hardness, low microporosity, among other properties[2]. Even though the improvement in the control of deposition parameters leads to better results of physical and chemical properties of the bulk material, this does not mean that the thin films are free of interconnected nanoporosity or nanocracks and it is important to correlate the process variables with the presence of these nanodefects[3].
In general, PVD, ion plating and sputtering deposition processes, involve atom-by-atom transfer mode. In the sputtering process, positive gas ions (Ar) produced in a glow discharge, bombard the target material (called cathode) and dislodging group of atoms, which then pass into the vapor phase and deposit onto the substrate. Physical characterization of thin films by image processing (including optical, scanning electron andatomic force microscopies, supported with micro and nanoindentation), could not predict the presence of interconnected nanodefects that reduce the lifetime of the coated parts, despite the fact that the coating materials themselves are highly corrosion resistant. Electrochemical Impedance Spectroscopy (EIS) is a powerful electrochemical technique and an interesting alternative to study the localized corrosion of coating/steel systems[4] where Electrical Equivalent Circuits (EECs) are built to afford an electrochemical description of the interface formed between the coating and the steel, as it changes during corrosion processes which occur in the system[5].
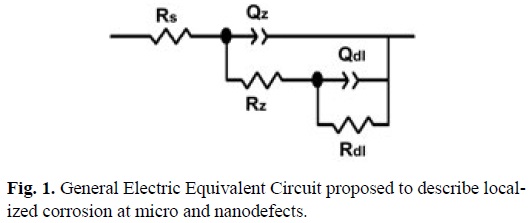
General EEC shown in fig. 1 is employed for modeling of EIS spectra of Ni-15wt%Cu coatings on steel, where Rs represent the electrical resistance of the electrolyte in series with Rz and QZ, representing the electrical resistance pore and the coating capacitance, respectively, in a parallel arrangement with Rdl and Qdl which are the polarization resistance and capacitance of substrate, respectively. The EEC employed in this paper has been proposed to describe the corrosion of PVD coated steel, where the substrate was corroded through nanodefects (pores and cracks).
2. Experimental Procedure
PVD Sputtering DC, model UV3 (Intercovamex) was used for the deposition of Ni-15wt%Cu coatings on steel as the base material; with a pressure of 2x10-3mbar, a flux of 18 c.c./min of argon, 20Khz of frequency, 100 watts of power and 5 and 12 minutes of deposition time. Rectangular test coupons of 25 x 30 mm were grounded with different grit sizes of SiC paper and polished with a diamond suspension. Impedance spectra of PVD coatings were obtained using a potentiostat-galvanostat ParStat 2273. EIS measurements were performed in 0.5 N NaCl solution at different times (24, 48 and 76 hours) in the 3-electrode mode using a saturated calomel reference electrode with a potential amplitude of 10mV, frequency range between 0.001mHz to 2MHz and 7 points per decade. The impedance spectra was collected as a function of exposition time and modeled using Zview 2.7 software to determine the corresponding EEC and the approximate values of the circuit elements fitted considering the experimental spectra. Deviation of the theoretical impedance from the experimental data was modeled with the Constant Phase Element "CPE" (Q), with significant improvement in the fit. After the electrochemical characterization, Scanning Electron Microscopy (SEM) was used to complement the characterization of the samples.
3. Results and discussion
Corrosion in PVD coatings is usually localized at the micro and nanodefects where the coating behaves like a capacitor (Fig. 1) and the electrolyte is able to contact the substrate through the pores [6,7].
Experimental EIS spectra were obtained from NiCu/Steel system, deposited with 100 watts of power with 5 and 12 >minutes of deposition time respectively. The coatings were exposed for different times, up to 76 hours in 0.5 N NaCl, to obtain the corresponding Bode plots and Nyquist diagrams. Fig. 2 shows the plots corresponding to the system NiCu-100Watts and 5 minutes of film deposition, which shows two time constants similar to two peaks after the first hour, corresponding with two parallel elements, (Qdl,Rdl ) at low frequencies (representing the substrate) and (Qz,Rz) at high frequency (corresponding to the coating response). Upon increasing the exposition time (24, 49 and 76 hours), only one time constant was observed at low frequency. Fits of the experimental spectra with the proposed EEC showed a good correlation (Table 1), where both constants a1 for the coating and a2 for the substrate, had values which correspond with the diffusion of reactive species, independent of the increase of resistivity R2 to the coating[8,9].
Fig.3. show the Bode plots corresponding to the system NiCu-100W at 12 minutes of film deposition, where one time constant time is observed at low frequencies, at the first hour of exposition in NaCl solution, and which again corresponds with the substrate response (Qdl,Rdl). Values obtained by fitting the experimental spectra are reported in Table 2, where both constants, a1 for the coating and a2 for the substrate, show values which correspond to the diffusion of reactive species, independent of the increase in resistivity Rz and Rdl for the coating and substrate respectively, similar to the case above described.
EEC established for both system NiCu-100Watts at 5 and 12 minutes, correspond with the general EEC for PVD coatings in Fig. 1; however, in this case and upon increases in the exposition times of the surfaces with NaCl solutions, there are more evident contributions of the substrate responses at low frequencies, indicating a higher corrosion rate, which is in turn localized in the pores and nanodefects of the main surface. In the system NiCu-100Watts at 5 minutes, the pore electrical resistance (Rz) shows a tendency to increase while the substrate resistance decreases. Due to the thickness of this coating (0.134 nm), the degradation of this thin film was fast, allowing the electrolyte to permeate and corrode the substrate. Micrographs shown in Fig. 4 show the coating delamination, exposing the substrate to the electrolyte through coating cracks.
In the system NiCu-100Watts at 12 minutes, both electrical resistances (pore- Rz and substrate- Rdl) increase with the exposition time, observing corrosion in both the coating and the substrate. The partial degradation of the thin film (0.243 yim thickness) allow that substrate oxides emerge and deposit over the coating, leading to an increase in the total resistance of the system, with a higher corrosion rate compared with the system NiCu -100Watts at 5 minutes. Micrographs in Fig. 5 show the corrosion products from the substrate deposited over the coating, increasing the resistance of the system.
Corrosion behavior of PVD films shows a permeability process through micro and nanodefects that increases as a function of time, until the electrolyte gets to the substrate, starting the corrosion process [10]. This permeability depends of the deposition parameters, affecting the film quality and could be correlated with the Bode diagrams [11].
During the delamination or degradation of the films, the total system resistance increases with a change in the capacitance values, resulting in one time constant, which corresponds with the substrate. In this case, the coating becomes coated with corrosion products from the substrate, increasing the system corrosion rate.
Conclusions
Nanodefects present in PVD coatings are detrimental to the corrosion resistance of such coated system; as permeable defects (e.g. pores and nanocracks) are particularly deleterious, providing a direct path for corrosive electrolytes to reach the coating/substrate interface. This permeation process induces galvanic corrosion started due to the difference in corrosion potentials between the PVD coating and the steel substrate. EIS allowed to study the localized corrosion and to evaluate the film kinetic degradation under corrosive medium, indicating that, during the first 24h of exposition, the electrolyte started the permeation process, partially affecting the film; at 48h, permeation is more evident due to the reduction of the film's resistance. After 76h of exposition, the system changes, starting the oxidation mechanism in the film that is represented with a one time constant in the Bode diagrams, progressing to general corrosion at the film surface [12].
Acknowledgements
The authors thank Consejo Nacional de Ciencia y Tecnología (CONACYT) from Mexico for the financial support in the project 135100 of basic research.
References
1. Rointan, F. B.; Handbook of Deposition Technologies for Films and Coating; University of California at Los Angeles, 2000. [ Links ]
2. Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. Corrosion Science. 2003, 54, 1243-1256. [ Links ]
3. Lee, Y. Y.; Enders, B.; Ensinger, W. Surface and Coatings Technology. 2002, 588-593. [ Links ]
4. Ismail, K. M.; Fathi, A. M.; Badawy, W. A. Corrosion Science, 2006, 48, 1912-1925. [ Links ]
5. Taylor, D.J.; Fleig, P.F.; Hietala, S.L. Technique for characterization of thin film porosity. Thin Solid Films. 1998, 332(1-2), 257-261. [ Links ]
6. Panjan, P.; et al. Vacuum. 2012, 86(6), 794-798. [ Links ]
7. Ahn, S.H.; et al. Surface and Coatings Technology. 2003, 162(2-3), 212-221. [ Links ]
8. Hummel, R. E. Understanding Materials Science: History, Properties, Applications, Second Edition, Springer, 2006. [ Links ]
9. Dourdain, S.; Mehdi, A.; Bardeau, J. F.;Gibaud, A. Thin Solid Films. 2006, 495, 205-209. [ Links ]
10. Brian, G. M. Surface coatings for protection against wear, in Surface coatings for protection against wear, W.p. limited, Editor. 2006, CRC Press LLC: Cambridge England. p. 429. [ Links ]
11. Wu, G., et al. Applied Surface Science. 2006, 252(20), 7422-7429. [ Links ]
12. Sahoo, P.; Das, S. K. Materials & Design 2011, 32, 1760-1775. [ Links ]














