Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.59 n.2 Ciudad de México Apr./Jun. 2015
Articles
Separation of Micro-Macrocomponent Systems: 149Pm-Nd, 161Tb-Gd, 166Ho-Dy and 177Lu-Yb by Extraction Chromatography
F. Monroy-Guzman and E. Jaime Salinas
Instituto Nacional de Investigaciones Nucleares, Carretera México-Toluca S/N, La Marquesa Ocoyoacac, 52750 Edo. de México, México. fabiola.monroy@inin.gob.mx
Received February 20th, 2015
Accepted April 4th, 2015
Abstract
149Pm, 161Tb, 166Ho, and 177Lu have advisable nuclear properties to be used in radiotherapy. They can be produced by neutron irradiation of a lanthanide target followed by β- decay and a posterior radiochemical separation of micro-amounts of daughter radionuclide from macro-amounts of the parent target. In order to accomplish the radiochemical separation of micro-macro systems: Nd/149Pm, Gd/161Tb, Dy/166Ho and Yb/177Lu, this work proposes the use of an extraction chromatographic with Ln SPS resin. Distribution coefficients and separation factors were determined and established the separation conditions of these micro-macro systems.
Key words: separation, extraction chromatography, 149Pm, 161Tb, 166Ho, 177Lu.
Resumen
149Pm, 166Ho, 161Tb y 177Lu poseen propiedades nucleares apropiadas para ser utilizados en medicina nuclear. Estos radiolantánidos pueden ser producidos por irradiación de neutrones del blanco, seguido de un decaimento β- y posteriormente la separación radioquímica de macro-cantidades de los blancos de las micro-cantidades de los radionuclides hijos producidos por decaimento. Con el fin de establecer la metodología de separación radioquímica de los sistemas micro-macro: Nd/149Pm, Gd/161Tb, Dy/166Ho y Yb/177Lu, se determinaron los coeficientes de distribución y factores de separación de estos sistemas en la resina Ln SPS. Se presentan las condiciones de separación de estos sistemas.
Palabras clave: separación, cromatografía extractiva, 149Pm, 161Tb, 166Ho, 177Lu.
1. Introduction
Radioactive lanthanides such as 149Pm, 161Tb, 166Ho and 177Lu, have a great potential in radiotherapy because they are beta or Auger-electron emitters with just enough gammas to enable imaging, with half-lives long enough to allow preparation and distribution of the radiopharmaceuticals, and can be prepared at high specific activities (carrier-free) [1,2].
Carrier-free radiolanthanides of high specific activity can be produced in nuclear reactors via neutron irradiation of massive lanthanide targets (>1 mg), as described in the reaction 1, followed by a radiochemical separation of the daughter radionuclide (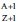 Ln,< 1µg) from the macro-amounts of the parent target (
Ln,< 1µg) from the macro-amounts of the parent target ( Ln)[3-9].
Ln)[3-9].
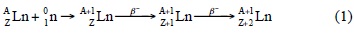
The difficulty in separating micro from macro-amounts of irradiated target are particularly arduous because lanthanides show great similarities in their chemical properties due to lanthanide contraction: the atomic size or ionic radii of tripositive lanthanide ions show a steady and gradual decrease with the increase in atomic number from La to Lu. A lot of works on lanthanides separation by liquid-liquid extraction have been reported and examined for improving the extraction and separation of individual rare earths, using as primary extractants: di(2-ethylhexyl)orthophosphoric acid (HDEHP), tributyl phosphate (TBP), carboxylic acids (naphthenic acids: n-hexanoic, n-octanoic, 3-cyclohexylpropanoic, iso-nonanoic, cyclohexanecarboxylic acids, 2-ethylhexanoic, 2-methyl-cyclohexanecarboxylic, 2,2,3-trimetylbutanoic, 2-butyl-2-ethylhexanoic, versatic, amines, etc. [10-12]. Nevertheless solvent extraction poses environmental problems because a large amount of organic solvent is inevitably used, due to the number of stages for solvent extraction cascades or batteries increases with the rise in purity of each individual rare earth produced. Further purification of micro-macro amounts of rare earths into the high ninety-nines purity are usually carried out by ion exchange and/or chromatographic techniques [1,5,9,10,13-15]. Ion exchange resins with alpha-hydroxyisobutyric acid (α-HIBA) have been currently used for lanthanides separation: however these procedures are either slow or give only partial separation or are unsuitable at high levels of activity [3,7,9, 12, 16,17]. For example, 166Ho has been separated from mg quantities of Dy target by a Aminex-A5 cation exchange and 0.085 M α-HIBA at pH = 4.3 as eluent, and purified with a cation exchange column from HCl solutions. This separation was achieved around 2 h, with a radiochemical yield of 95% and a radionuclide purity 166Ho of 99.9 % [1,9]. 177Lu from Yb target has been performed by Na(Hg) amalgam from Cl-/CH3COO- electrolytes, followed by a final cation exchange purification. The cementation separation process provides a decontamination factor of Yb(III) of 104, the cation exchange purification adding a decontamination factor of >102 and is performed within 4-5 h. The radiochemical yield of this process is about 75% [6]. In the case of 161Tb, an isolation of 80 to 90% of 161Tb from massive 160Gd targets with a decontamination factor from gadolinium >105 has been achieved using a cation exchange resin Aminex A6 eluted with α-HIBA at ph 4.5 and purified with a Bio-Rad AG 50W-X8 cation exchanger to produce a 0.04 M HCl solution [3].
The extraction chromatography column is becoming widely advantageous in the separation of very similar ions such as lanthanides because it couples the favorable selectivity features of the organic compounds used in liquid-liquid extraction, with the multistage character of a chromatographic process [18]. In addition, extraction chromatography is a fast, efficient technique and generates less waste than other separation technique. The organic phosphorus compounds that are most frequently used as stationary phases in the lanthanides separation are HDEHP, TBP, Diphenyl(dialkylcarbamoylmethyl) phosphine oxides, etc., however the best separation factors (> 2.5) between adjacent rare earths are obtained with HDEHP as stationary phase fixed in Kiselguhr, Corvic (poly(vinyl chloride-vinyl acetate)copolymer), Kel-F (polychlorotrifluoroethane), Zorbax-SIL,(porous silica), PMS (polymethysilane) or Celite® (diatomaceous earth) and as mobile phases solutions containing acids (HNO3, HCl, HClO4) [1,5, 13,19-22]. In general, the separations of all the rare earth elements at a tracer level on HDEHP-solid support are satisfactory by gradient elution; however some difficulties have been reported in the separation of consecutive lanthanides such as: Nd-Pm, Dy-Ho and Yb-Lu depending on the type of support used and the column length [20-22]. Ketring performed a very neat separation of Nd/Pm, Dy/Ho and Yb/Lu using the Ln spec resin (50-100 μm) from Eichrom, formed by HDEHP (40 % by weight) supported on an inert polymeric absorbent (60 % by weight), nervertheless the precise conditions of these separations have not been reported [5].
The objective of this work was to establish a radiochemical method to separate the carrier free radiolanthanides: 149Pm, 161Tb, 66Ho and 177Lu from irradiated natural targets (Nd, Gd, Dy and Yb), using an extraction chromatographic resin. Horwitz reported distribution coefficients (Kd) versus nitric acid only for Pm, Gd, Tb and Lu using HDEHP on a hydrophobic support, thus distribution coefficients for Nd, Dy, Ho and Yb were determined in this work as well as those of Pm, Gd, Tb and Lu under our experimental conditions and compared with the values reported by Hotwitz [19].
2. Results
2.1. Distribution Coefficients of Nd, Pm, Gd, Tb, Dy, Ho, Yb and Lu
Figure 1 shows the Kd values of Nd, Pm, Gd, Tb, Dy, Ho, Yb and Lu as a function of HNO3 concentration in Ln SPS Eichrom resin. The Kd values decrease with an increase in HNO3 solution concentration and a diminution of the lanthanide atomic number at a fixed HNO3 concentration. For example, Dysprosium (Z=66) at 3 mol/L HNO3 has a Kd value of 26 cm3/g while Holmium (Z=67), Yterbium (Z=70) and Lutetium (Z=71), at the same HNO3 concentration, present Kd values of 37, 310 and 707 cm3/g, respectively. Thus, the parent lanthanide of the parent/daughter pairs: Nd/Pm, Gd/Tb, Dy/Ho and Yb/Lu, will be eluted at first during the chromatographic separation process. The Kd values of Pm, Gd, Tb and Lu are in good agreement with those reported by Hotwitz [19].
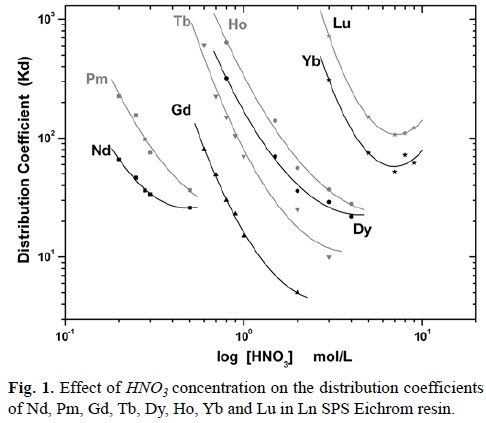
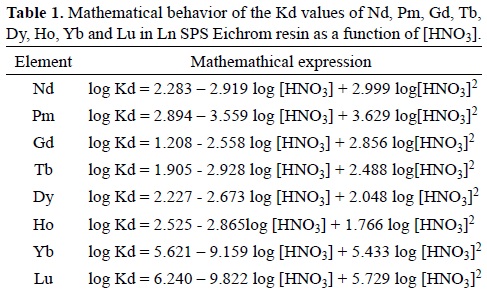
Table 1 presents the mathematical equations that express the behavior of the Kd values of Nd, Pm, Gd, Tb, Dy, Ho, Yb and Lu shown in Figure 1, which present an parabolic behavior.
2.2. Separation factors (α) of pairs: Nd/Pm, Gd/Tb, Dy/Ho and Yb/Lu
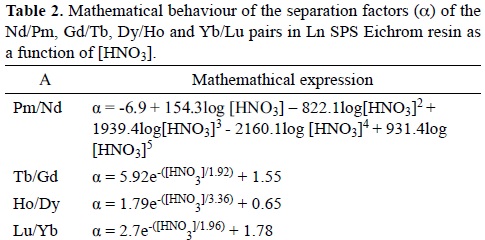
The separation factors (α) of the Nd/Pm, Gd/Tb, Dy/Ho and Yb/Lu pairs were calculated from their Kd values by using the mathematical expressions depicted in Table 1. The α values obtained then were plotted as a function of HNO3 concentration (Figure 2) and their respective mathematical equations are shown in Table 2.
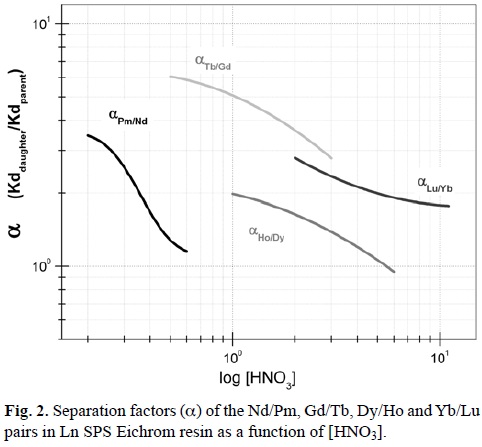
The α value determines the separation performance of the pairs. When α is equal to one, the Kd values of both elements are identical and they are not able be separated. So, the greater the α value, the more efficient the separation and the higher purity of the radioisotope isolated is obtained. Additionally, the α values of the pairs studied decrease with the increase of the HNO3 concentration. It follows that the slopes of α Daughter/Parent functions define if α decreases rapidly (αPm/Nd) or slowly (αLu/Yb). The separation factors of the pair Tb/Gd are the highest and those for Ho/Dy lower. Tb and Gd separation have a better resolution that that of Ho/Dy or Lu/Yb.
2.3 Selection of separation conditions of the Nd/Pm, Gd/Tb, Dy/Ho and Yb/Lu pairs
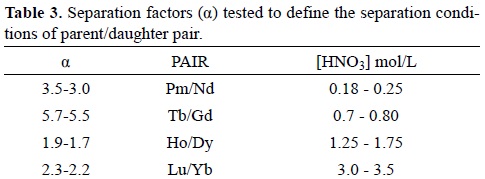
In principle, it is feasible to separate the Nd/Pm, Gd/Tb, Dy/Ho and Yb/Lu pairs if α value (See Figure 2) is greater than 1. Therefore, the best separation performances are essentially obtained with high αDaughter/Parent values (>>1); however it is also important to consider the HNO3 volume used to elute lanthanides, which is closely dependent on the Kd values. Distribution coefficients higher than 200 impose the use of large volumes of eluent and also require more time to achieve an efficient separation. For this reason, the separation conditions of the Nd/Pm, Gd/Tb, Dy/Ho and Yb/Lu pairs were selected when higher separation factor (α) values at Kd values lower than 200 were found. HNO3 concentration ranges chosen to select the separation conditions for each parent/daughter pair are shown in Table 3.
2.4 Separation of the Nd/Pm, Gd/Tb, Dy/Ho and Yb/Lu pairs
The separation tests of the parent/daughter pairs at HNO3 concentrations stated in Table 3, were determined following the same methodology described in section 4.1. The separation efficiencies of the parent/daughter pairs and the radionuclide purity of daughters obtained are shown in Table 4. Highest separation efficiencies and radionuclide purities of more than 99.9% were selected as the optimal separation conditions of each parent/daughter pair. Thus, the separation conditions selected for each pair are shown bolded in Table 4 and the respective elution profiles in Figure 3. Gadolinium and Terbium were separated with an efficiency of 100% using 22 mL of 0.8 mol/L HNO3 and 18mL of 3 mol/L HNO3 to recover Gd and 161Tb respectively, with a 100% radionuclide purity for 161Tb. In the case of Neodymium and Promethium, the use of 106 mL and 30 mL of 0.18 and 1.5 mol/L HNO3, respectively, is required to achieve a separation efficiency of 98.4 % and a 149Pm radionuclide purity of 99.9%. Dysprosium and Holmium separation was attained with an efficiency of 100% by using 50 mL and 35 mL of 1.4 and 3 mol/L HNO3 respectively, and 100% pure166Ho was obtained. Finally, Ytterbium and Lutetium were separated with an efficiency of 89.7% by using 165 mL of 3.4 mol/L HNO3 to obtain Ytterbium and 55 mL of 8 mol/L HNO3 to recover the 177Lu at a 99.9% radionuclide purity. In order to get a 177Lu radionuclide purity higher than 99.9%, the first 6 mL of the 177Lu eluate were removed (See Figure 3).
3. Discussion
The production of carrier-free 149Pm, 161Tb, 166Ho and 177Lu is based on the neutron irradiation of 148Nd, 160Gd, 164Dy and 176Yb enriched targets in nuclear reactors, followed by a radiochemical separation of the 149Pm, 161Tb, 166Ho and 177Lu from the macro-amounts of the neodymium, gadolinium, dysprosium and ytterbium targets [3-9]. However, Nd, Gd, Dy and Yb natural targets were irradiated in this work, producing the nuclear reactions listed in table 5. The radioisotopes used to follow the separation processes of Nd/Pm, Gd/Tb, Dy/Ho and Yb/Lu pairs and to determine the distribution coefficients of these lanthanides, were selected in accordance with the following criteria:
(A) For Neodymium/Promethium: Nd-147 (11 d), Nd-149 (1.7 h) and Nd-151 (12.4 min) are produced after 3 h of irradiation; Nd-151 and Nd-149 disappear after a decay time of 14 h. Thus Kd determinations and Nd/Pm separation process were performed with Nd-147. Pm can be evaluated with any radioisotopes produced by decay (Pm-147, 149 and 151); however the best option is Pm -151 at 340 keV with a relative intensity of 100% because the low relative intensity of gamma-rays emitted by Pm-147 and Pm-149 (<0.01%).
(B) For Gadolinium/Terbium: target irradiation for 15 minutes produces Gd-153 (241.6 d), Gd-159 (18.6 h) and Gd-161(3.2 min). The latter disappears after 30 min and Gd-153 requires longer counting times for its half-life, therefore Gd-159 was chosen and measured at 363 keV (100%). Only one terbium radioisotope is produced by the Gd irradiation and its decay: Tb-161.
(C) For Dysprosium/Holmium: Dy-157 (8.2 h), Dy-159 (134 d), Dy-165(1.25 min) and Dy-166 are produced during irradiation of dysprosium target. Dy-165 decays at 10 minutes, Dy-159 activity produced is very low and requires long counting time, while the 82 keV gamma-ray of Dy-166 can interfered with the gamma-rays emitted by Ho-166, therefore, Dy-157 was selected and counted at 326 keV (100%). Ho-166 is the only one holmium radioisotope generated.
(D) For Ytterbium/Lutetium: ytterbium radioisotopes produced are: Yb-169 (32 d), Yb-175 (4.2 d) and Yb-177 (1.9 h) and after 7 d decay to generate Lu-177, Yb-177 disappears and Yb-175 decays considerably. Lu-177 is the only one lutetium radioisotope generated in the irradiation-decay process. Therefore Yb-169 and Lu-177 were selected to follow Yb/Lu separation process.
One of the mechanisms postulated for the extraction adduct between a lanthanide ion and HDEHP (reaction 2) can be divided into two steps: the formation of extractable species in aqueous phase (reaction 3), and its partition between the two phases (reaction 4), considering that HDEHP exists as a dimmer [18]:
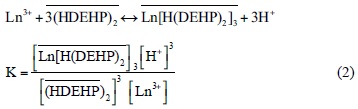
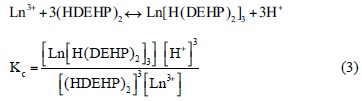

(barred symbols denote the organic phase and Ln lanthanides (III)).
The distribution ratio of lanthanides can be given as:

Thus, the two essential factors determining the magnitude of distribution coefficient are: complex formation and the partition between the two phases. Extraction is greater for that lanthanide which more readily forms an extractable complex can be then expressed as a function of the partition coefficient (P) and the stability constant of the complex (Kc), substituting the constants into the above equation yields the following simplified form:
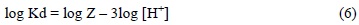
In accordance with expression 6, the lanthanides's distribution coefficients follow a linear function form, where the constant and the linear coefficient correspond to logZ, where Z represents the sum of P, Kc and HDEHP concentration, considering that HDHEP is constant, and -3log[H+] (equation 6) respectively. However, the mathematical expressions of lanthanides's Kd, shown in Table 1, correspond to quadratic functions where the constant and the linear coefficient agree to logZ and -3log[H+] of the equation 6; while the quadratic coefficient constants, positive for all lanthanides, indicate that the Ln[H(DEPH)2]3 complex dissociates in Ln3+ and HDEHP at high HNO3 and thus Kd values decrease. It is important to note that these Kd values reach to a minimum even if the acid concentration is very high, because the full complex dissociation is never reached.
On the other hand, in the case of Yb and Lu, the logP values and the quadratic coefficient constants are approximately double that for the rest of lanthanides, while their linear coefficient constants are three times greater than that obtained for the other lanthanides.
The extractability of HDEHP is highly dependent upon the acidity of the aqueous phase, and the distribution coefficients of lanthanides substantially increase with Z and the concentration of the nitric acid (See Figure 1) because the changes in the stability constant with Z. Larger stability constant for the complex Ln[H(DEHP)2]3, results in a larger concentration of extractable species in the aqueous phase and consequently a larger distribution coefficient. Therefore, the order of elution of consecutive lanthanides is proportional to Z: Nd is eluted first Pm, Gd first Tb, etc. (see Figure 3). This order favors the separation between micro-amounts of the daughter radioisotope from the macro-amounts of lanthanide target because the chromatographic column retains the micro-component while the macro-component is in the meantime eluted away. However, when a stationary phase is loaded with macro-amounts of retained elements, variations of its retention features may often occur. Distribution coefficients usually are referred to the extraction of micro or even tracer amounts of metals and it is known that the behaviour of many extractants at such metal levels can be quite different from that shown at high organic phase loading conditions [18]. Thus, the distribution coefficients of Nd, Pm, Gd, Tb, Dy, Ho, Yb and Lu were determined under the same conditions used in the separation of pairs: Nd/Pm, Gd/Tb, Dy/Ho and Yb/Lu. These values are in accordance with those reported by Horwitz et. al. for Pm, Gd, Tb and Lu, using undiluted HDEHP on Celite at 50°C [19].
Distribution coefficients are “suitable" for chromatographic separations of lanthanides not only when they result in an advantageous separation factor for the neighbor lanthanides, but also when they are not so low or high so as to prevent the separation of elements or their recovery into acceptable eluent volumes [18]. Therefore, the values of the distribution coefficients selected to desorb the daughter radioisotopes: 149Pm, 161Tb and 166Ho were less than 20 in order to recover it at the smallest possible volume. In the case of 177Lu, the minimum value of Kd is about 100 as shown in Figure 1, for this reason its eluate volume is higher than 50 mL.
As regards the mathematical function of alpha (See Table 2), it is important to note that: Exponential and polynomial models were applied to alpha data in order to find the mathematical function that gives the best approximation to our results. All alpha data fit with the exponential model presented a SSR > 0.999, however a best approximation of Nd/Pm data (SSR=1) were obtained in the polynomial model. This difference could be explained in terms of lanthanide contraction, which causes a decrease in atomic and ionic size from La to Lu. Therefore, the chemical properties of Nd and Pm (light lanthanides) in aqueous solution vary slightly to those of Dy to Lu (heavy lanthanides).
4. Conclusions
The method proposed in this work to separate micro-amounts of 149Pm, 161Tb, 166Ho and 177Lu, produced via a neutron capture followed by β- decay:  Ln, (n,γ)
Ln, (n,γ)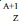 Ln (β-,γ)
Ln (β-,γ)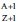 Ln, from macro-amounts of the irradiated target (Nd, Gd, Dy or Yb) is based on extraction chromatographic using Ln SPS resin and HNO3 solutions as mobile phase.
Ln, from macro-amounts of the irradiated target (Nd, Gd, Dy or Yb) is based on extraction chromatographic using Ln SPS resin and HNO3 solutions as mobile phase.
The distribution coefficients of lanthanides in Ln SPS resin decrease with an increase in HNO3 solution concentration and a diminution of the lanthanide atomic number at a fixed HNO3 concentration. Thus, the parent lanthanides of the parent/daughter pairs: Nd/Pm, Gd/Tb, Dy/Ho and Yb/Lu are eluted at first during the chromatographic separation process.
The best separation performances of the parent/daughter pairs: Nd/Pm, Gd/Tb, Dy/Ho and Yb/Lu were obtained with high αDaughter/Parent values (>>1) at Kd values lower than 200, using the following conditions: for Neodymium and Promethium use 0.18 and 1.5 M HNO3, for Gadolinium and Terbium use 0.8 and 3 M HNO3, for Dysprosium and Holmium use 1.5 M HNO3 and for Ytterbium and Lutetium use 3.4 and 8 M HNO3 respectively.
5. Experimental
The distribution coefficients (Kd) of Nd, Pm, Gd, Tb, Dy, Ho, Yb and Lu in Ln SPS Eichrom resin from Eichrom Darien were determined for dynamic conditions by radiotracer technique using 147Nd, 149Pm, 159Gd, 161Tb, 157Dy, 166Ho, 169Yb and 177Lu (See Table 6) .
Radiolanthanides were produced by irradiation of 10 mg Nd2(NO)3, Gd2(NO)3, Dy2(NO)3 and Yb2(NO)3 in the TRIGA MARK III Reactor of the National Institute of the Nuclear Research (ININ) in Mexico, to a neutron fluence rate of 1.6 x1012 n cm-2s1. Neodymium and dysprosium salts were irradiated for 3 h, ytterbium for 20 min and gadolinium for 15 min. 147Nd, 159Gd, 157Dy and 169Yb were produced by neutron irradiation of the nitrate salts, while 151Pm, 161Tb, 166Ho and 177Lu were formed from radioactive nitrate salt decay after 14 h, 3 min, 16 h and 7 d respectively. The radioactive nitrate salts were dissolved in 300 µL of 0.15 mol/L HNO3, to generate 0.098 mol/L solutions with a specific activity of 0.148 kBq/µL.
The Kd measurements were determined using glass chromatographic columns (12 mm × 70 mm) loaded with 2 g of Ln spec resin (50-100 µm) from Eichrom Industries of Darien, IL (USA) previously preconditioned with 0.15 mol/L HNO3. The Ln spec resin is comprised of a solution of di(2-ethylhexyl)orthophosphoric acid (HDEHP) (40% by weight) loaded onto the inert polymeric absorbent (60% by weight) Amberchrom™ CG-71.
All experiments were performed in the elution mode process. Radiolanthanide solutions (~100 μL) were loaded on the column which was then eluted with the selected medium, HNO3 solutions at different concentrations. Elution profiles were obtained by collecting each fraction measured under a coaxial gamma detector HPGe (Canberra 7229P) connected to a PC-multichannel analyzer (ACCUSSPECT-A, Canberra). Gamma-ray spectra were analyzed using the gamma software for “Genie 2000" Canberra Acquisition and Analysis with fixed geometry and varied counting time between 200 and 500 seconds. Table 6 shows the corresponding photopeaks used to calculate the Kd values for 147Nd, 151Pm, 159Gd, 161Tb, 157Dy, 166Ho, 169Yb and 177Lu [23].
The Kd values were calculated using the relation:
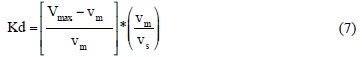
where Vmax is the eluate volume to peak maximum or retention volume, vm is the void volume or the volume of the mobile phase, vs is the volume of the stationary phase.
The Kd values were used to determine the separation conditions of the Parent/Daughter pairs (Nd/Pm, Gb/Tb, Dy/Ho and Yb/Lu) by calculating the separation factor or selectivity (α), which is defined as the ratio of the distribution coefficients of two adsorbed solutes measured under the same conditions:

where KdP and KdD are the Kd values of the parent and daughter element respectively.
The separation factor can be visualized as the distance between the apices of the two chromatographic peaks. High α values indicate good separation power.
Acknowledgements
This work was supported by the CONACYT-SALUD-2004-C01-001. The authors indebted to the technical staff of reactor Triga Mark III (México): Maximiano Hernández, Wenceslao Nava, Margarito Alva, Braulio Ortega, Edgar Herrera and Roberto Raya.
6. References
1. Nayak, D.; Lahiri S. J. Radioanal. Nucl. Chem. 1999, 242, 423-432. [ Links ]
2. Uusijarvi, H.; Bernhardt, P.; Rosch, F.; Maecke, H.R.; Forssell-Aronsson, E. J. Nucl. Med. 2006, 47, 807-814. [ Links ]
3. Lehenberger, S.; Barkhausen, C.; Cohrs, S.; Fischer, E.; Grunberg, J.; Hohn, A.; Koster, U.; Schibli, R.; Turler, A.; Zhernosekov, K. Nucl. Med. Biol. 2011, 38, 917-924. [ Links ]
4. Godoy, N. O.; Pinto, L. N.; Avila, M. J. Alasbimn J. 2002, 5,1-2. [ Links ]
5. Ketring. R.; Ehrhardt, G. J.; Embree, M. F.; Bailey, K. D.; Tyler, T. T.; Gawenis, J. A.; Jurisson, S. S.; Engelbrecht, H. P.; Smith, C. J.; Cuttler, C. S. Alasbimn J. 2003, 5,1-6. [ Links ]
6. Lebedev, N. A.; Novgorodov, A. F.; Misiak, R.; Brockmann, J.; Rösch, F. Appl. Radiat. Isot. 2000, 53,421-425. [ Links ]
7. Dadachova, E.; Mirzadeh, S.; Smith, S. V.; Knapp, F.F.; Hetherington, E.L. Appl. Radiat. Isot. 1997, 48, 477-481. [ Links ]
8. Pillai, M. R. A.; Chakraborty, S.; Das, T.; Venkatesh, M.; Ramamoorthy. Appl. Radiat. Isot. 2003, 59, 109-118. [ Links ]
9. Dadachova, E.; Mirzadeh, S.; Lambrecht, R.M.; Hetherington, E.L.; Knapp, F.F. (Jr.). Anal. Chem. 1994, 66, 4272-4277. [ Links ]
10. Gschneidner, K. A.; Hardbound, L. E., in: Handbook on the Physics and Chemistry of Rare. Bünzli, J. C., Pecharsky, V. Ed. Elsevier North, Holland, 2000, 432-455. [ Links ]
11. Moore, F. L. Anal. Chem. 1965, 37, 1235-1239. [ Links ]
12. Nayak, D.; Lahir, S. Solv. Ext. Ion. Exch. 1999, 15, 1133-1154. [ Links ]
13. Horwitz, E. P.; Bloomquist, C. A. A.; Delphin, W. H. J. Chrom. Sci. 1977, 15, 41-46. [ Links ]
14. Russ, K. Jr. Radiopharmaceuticals for Nuclear Medicine and Oncology- The Central Role of Chemistry. XLI Congreso Mexicano de Química, Mexico City, 2006, 1-6. [ Links ]
15. Le Naour, D. T.; Brillard, L.; Hussonnois, M.; Monroy-Guzmán, F.; Constantinescu, O.; Le Du, D.; Meunier, R. Radiochim. Acta. 1997, 77, 143-148. [ Links ]
16. Chopin, G. R.; Silva, R. J. J. Inorg. Nucl. Chem. 1956, 3, 153-154. [ Links ]
17. Chopin, G. R.; Chopoorina, J. A. J. Inorg. Nucl. Chem. 1961, 22, 97-101. [ Links ]
18. Braun, T.; Ghersini, G., Eds. Extraction Chromatography. Elsevier: Hungary, 1975. [ Links ]
19. Horwitz, E.P.; Bloomquist, C.A.A. J. Inorg. Nucl. Chem. 1975, 37, 425-434. [ Links ]
20. Cerrai, E.; Testa, C. J. Inorg. Nucl. Chem. 1963, 25, 1045-1050. [ Links ]
21. Turanov, A. N.; Karandashev, V. K.; Yarkevich, A. N.; Kharitonov, A. V.; Safronova, Z. V. R. Radiochemistry. 2002, 44, 559-564. [ Links ]
22. Sochacka, R. J.; Siekierski, S. J. Chromatography. 1964, 16, 376-384. [ Links ]
23. Erdtmann, G.; Soyka, W. The Gamma Rays of the Radionuclides: Tables for Applied Gamma Ray Spectrometry. Verlag Chemie, Germany, 1979. [ Links ]














