Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.59 n.2 Ciudad de México Apr./Jun. 2015
Articles
Role of Different Transporting Systems in the Secretion of Alkaloids by Hairy Roots of Catharanthus roseus (L) G. Don
Juan Luis Monribot-Villanueva,1,3 Eliel Ruiz-May,1,3 Rosa María Galaz-Ávalos,1 Dayakar Badri2 and Víctor Manuel Loyola-Vargas*1
1 Unidad de Bioquímica y Biología Molecular de Plantas, Centro de Investigación Científica de Yucatán. Calle 43 No. 130, Col. Chuburná de Hidalgo, Mérida, Yucatán, México.vmloyola@cicy.mx Tel.: 52-999-9428330.
2 Center for Rhizosphere Biology, Colorado State University, Fort Collins, CO 80523, USA.
3 Current address: Red de Estudios Moleculares Avanzados, Cluster Biomimic®, Instituto de Ecología A.C., Carretera Antigua a Coatepec No. 351, Congregación el Haya, CP 91070, Xalapa, Veracruz, México.
Received October 27th, 2014
Accepted February 17th, 2015
Abstract
Knowledge on the biosynthetic pathways of the monoterpene alkaloids is enormous, but little is known about their mechanism of transporting system from the plant cell. There is not concrete evidence confirming the role of ABC transporters in the secretion of monoterpene indole alkaloids (MIAs) in Catharanthus roseus. Therefore, in order to determine the role of different transporting systems involved in the MIAs translocation, we employed a pharmacological approach by using transport inhibitors such as, KCN, Na3VO4, quinidine and glibenclamide in hairy root cultures of C. roseus. It was found that the accumulation of ATP drastically decreased in the presence of KCN or 100 µM acetylsalicylic acid (ASA)/100 µM KCN. The treatment with the inhibitors KCN and glibenclamide in the presence of ASA significantly increased the ajmalicine secretion compared to the control. The secretion of serpentine was undetected during the first 24 h in all the samples. Treatment with the inhibitors quinidine and glibenclamide provoked a significant reduction of serpentine secretion in the hairy roots compared to the control. Based on our results, we found evidence that ABC transporters might participate in the secretion of MIAs by C. roseus hairy roots.
Key words: ABC transporters; alkaloids; Catharanthus roseus; exudation; inhibitors.
Resumen
El conocimiento de las rutas de biosíntesis de los alcaloides monoterpénindólicos (AMIs) es amplio, pero prácticamente no se conoce nada sobre los mecanismos de transporte que se requieren para que dichas rutas funcionen adecuadamente. Puesto que sólo se ha descubierto uno de estos transportadores, perteneciente a la familia de transportadores ABC, en la investigación reportada en este artículo presentamos evidencia de la participación de tales transportadores utilizando un acercamiento farmacológico mediante el uso de los inhibidores KCN, Na3VO4, quinidina y glibenclamida durante la secreción de AMIs en raíces transformadas de Catharanthus roseus bajo condiciones de inducción y no inducción. La acumulación de ATP disminuye drásticamente en presencia de KCN o 100 µM ácido acetil salicílico (AAS)/100 µM KCN. El tratamiento con los inhibidores KCN y glibenclamida, en presencia de AAS, aumenta significativamente la secreción de ajmalicina, comparada con el testigo. La secreción de serpentina fue indetectable durante las primeras 24 h en todas las muestras. Las raíces en el tratamiento con los inhibidores quinidina y glibenclamida mostraron una significativa disminución en la secreción de serpentina comparada con el testigo. Nuestros resultados muestran evidencia de que los transportadores ABC pueden estar participando en la secreción de AMIs por las raíces transformadas de C. roseus.
Palabras clave: alcaloides; Catharanthus roseus; inhibidores; transportadores ABC; secreción.
Introduction
Catharanthus roseus synthesizes more than 140 secologanin-derived monoterpene indole alkaloids (MIAs) [1], of the approximately 2,000 known compounds, and some of them such as ajmalicine, vincamine and reserpine (peripheral vasodilators), ajmaline (antiarrhythmic), vinblastine and vincristine (anticancer), and yohimbine (pro-erectile) possess important pharmacological effects [2] widely used in medicine. The biosynthesis of these compounds involves a very complex pathway and also requires the participation of different cell types and different organelles within the cell [3, 4]. These pathways require of a very precise transport system.
A pioneer study reported the transport of vindoline and ajmalicine by a specific proton-antiporter system [5]. Followed by this report, another study demonstrated that the transport of vindoline is carried out by an energy-dependent transporter by using C. roseus protoplasts [6], besides an ion-trap mechanism that could contribute to the vacuolar uptake of these endogenous alkaloids [7]. Recently, it was demonstrated that vacuolar transport of MIAs is mediated by a proton-driven antiport and not by an ion-trap mechanism or ABC transporters [8]. However, the expression of the CjMDR1 gene, a transmembrane ABC transporter from Coptis japonica, in C. roseus cells, produced an increase in the accumulation of ajmalicine and tetrahydroalstonine, alkaloids from C. roseus but not of berberine, the main substrate of the CjMDR1 transporter [9]. Recently, the screening of the Plant Medicinal Genomics Resource database allowed to El-Guizani et al. [10] to identify 16 ABC transporters partial sequences in C. roseus.
The alkaloids biosynthetic pathway can be regulated at the cellular and at the molecular level [11], which includes their transport through the membranes of different organelles and different tissues. The De Luca's laboratory has found that vindoline is accumulated inside the leaf cells, meanwhile catharanthine accumulates in leaf wax exudates [12]. This separation is mediated by a catharanthine transporter (CrTPT2) that is present in the epidermis of young leaves [13]. The secretion of alkaloids to the leaves surface may be mediated by unique transporters to MIA-producing plant species. A strong candidate could be the transporters involved in the cuticle assembly, which are of the kind of ABC transporters.
Previously, it was reported the accumulation and secretion of MIA by treatment with different elicitors (methyl jasmonate, acetyl salicylic acid and nitric oxide) in in vitro tissue cultures of C. roseus, such as hairy roots [14, 15], cell suspensions [16] and tumor lines [17]. In our laboratory, we observed that the hairy roots of C. roseus elicited with methyl jasmonate (MeJA) showed a differential secretion of ajmalicine, serpentine, ajmaline and catharanthine compared to the controls [15]. This treatment also increased the accumulation of H2O2 during the first 48 h [18].
It has been demonstrated the involvement of ABC transporters in the root secretion of phytochemicals [19-22]. In agreement with these studies, recently it has been showed a tight regulation in the export and accumulation of defense phytochemicals in the rhizosphere [23, 24]. In addition, the ABC transporter, GmPDR12 expression was induced in response to salicylic acid and MeJA [19]. Differential expression of ABC transporters in Arabidoposis thaliana root tissues was observed in response to nitric oxide, salicylic acid and MeJA [22]. Based on these observations, one can hypothesize that ABC transporters might be involved in transporting MIAs from C. roseus cell cultures.
Better understanding about the transport mechanism of secondary metabolites and their regulatory networks would provide the development of novel methods to engineer C. roseus plants for commercial applications. In the present study, we aimed to identify the type of transporters for MIAs by employing pharmacological approach using C. roseus hairy root cultures.
Results
To test whether the secretion of alkaloids is facilitated by ABC transporters, we employed a pharmacological approach by using inhibitors of different transporting systems. If the secretion of MIAs is mediated by ABC transporters, ATP must play a central role and inhibition of ATP production must modify the exudation process.
Effect of inhibitors in plant growth and ATP accumulation
To determine the effect of inhibitors on hairy roots growth, we treated the hairy roots with 100 mm of all inhibitors independently and dry weight (DW) was analyzed. We did not observe any difference in the dry weight after 48 h of all inhibitors treatment but significant reduction on the DW of hairy roots was observed after 72 hours only in 100 µM ASA treatment (Fig. 1A).

We also analyzed the ATP levels in response to the inhibitors. The accumulation of ATP drastically decreased (close to zero) in the presence of KCN or 100 µM ASA/100 µM KCN (Fig. 1B) and the condition was lasted for the next 60 h. The treatment with 100 µM ASA alone slightly decreased the accumulation of ATP during the first 36 h and slowly the ATP levels were increased to 70 µg ATP g-1 fresh weight (FW) (Fig. 1B), while the control remains around 40 µg ATP g-1 FW after an increase during the first 36 h. High significant differences were observed in the levels of ATP between the treatments of 100 mM ASA and KCN (Fig. 1B).
Effect of different transporting systems inhibitors in ajmalicine secretion by hairy roots of C. roseus.
Ajmalicine was the more abundant MIA identified in the culture medium of hairy roots. The secretion of this alkaloid was significantly increased after 48 h of the treatment with 100 µM ASA (Fig. 2A) and the level of ajmalicine was two-fold more (7.75 mg L-1) than the control (3.06 mg L-1) after 72 h of treatment. In the presence of inhibitors alone (KCN, orthovanadate, quinidine and glibenclamide), we did not observe any significant changes in ajmalicine secretion compared to control. But, we observed differential changes in ajmalicine secretion when hairy roots treated simultaneously with the inhibitor and ASA. For instance, treatment with the inhibitors, KCN (Fig. 2A) and glibenclamide (Fig. 2D) in the presence of ASA significantly increased the ajmalicine secretion compared to control. Unlikely, the inhibitors orthovanadate (Fig. 2B) and quinidine (Fig. 2C) in the presence of ASA did not show any significant changes in ajmalicine secretion.
Effect of inhibitors in serpentine secretion by hairy roots of C. roseus
The secretion of serpentine was undetected during the first 24 h in all the samples (Figs. 3A-3D). In the control samples there was a gradual increase in the secretion of serpentine (0.8 mg L-1) at 48 h (Figs. 3A-3D). In the presence of ASA, the serpentine secretion was increased to 2.0 mg L-1 after 24 h and stayed high till 72 h period of the study (Fig. 3A). When treated with the inhibitors alone we observed different trends: KCN did not show significant change compared to control (Fig. 3A) and unlikely orthovanadate showed significant increase in serpentine secretion compared to control at 48 h (Fig. 3B). On the contrary, the inhibitors quinidine and glibenclamide showed significant reduction of serpentine secretion compared to control (Figs. 3C-3D). Interestingly, the treatment with quinidine in the presence of ASA also showed significant reduction in serpentine secretion at 72 h (Fig. 3C) but the presence of ASA with other inhibitors showed significant increase in serpentine secretion (Figs. 3A-3D).

Effect of inhibitors in catharanthine secretion by hairy roots of C. roseus
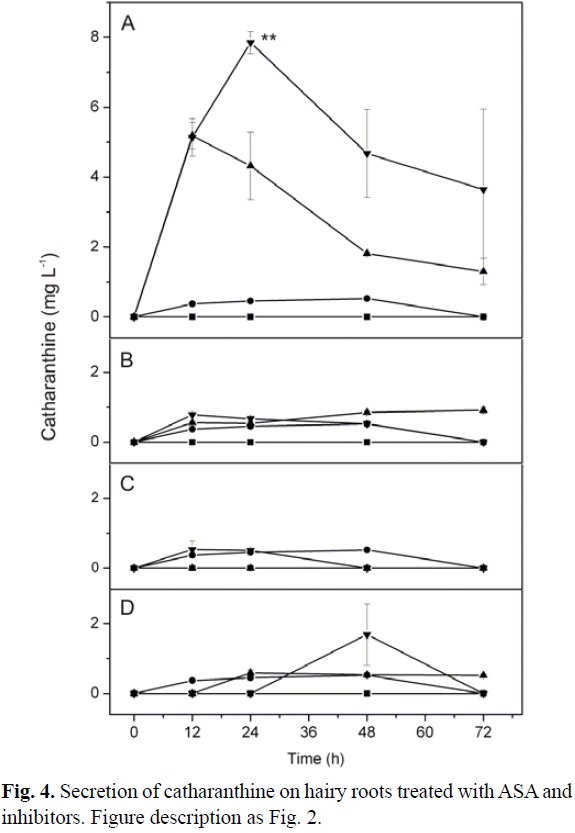
Catharanthine secretion did not show significant difference between the treatments with 100 µM ASA and the control (Fig. 4A-4D). However, the treatment with 100 µM KCN alone, significantly increased the secretion of catharanthine at 24 h (Fig. 4A). Furthermore, the treatment of hairy roots with 100 µM ASA/100 µM KCN significantly increased catharanthine secretion in the culture media in comparison with other treatments and control (Fig. 4A). But, the treatment of hairy roots with other inhibitors (orthovanadate, quinidine and glibenclamide) in the presence and absence of ASA did not show any significant changes in catharanthine secretion compared to control (Fig. 4B-4D).
Non-target profile of secreted compounds by hairy roots of C. roseus in the presence of inhibitors
The treatment of C. roseus hairy roots with 100 µM ASA increased the secretion of several unidentified compounds (Table 1; peaks 1, 4, 9 and 21) along the 72 h of observation (Tables 1, 3, 5, 7). After 12 h of treatment with 100 µM KCN, it was observed the decrease of the peaks 2, 6 and 13 (Table 1); same for peaks 1, 2 and 13 at 24 h of treatment (Table 3). The treatment with ASA/KCN reduced the secretion of compounds 2, 5 and 13 during the first 12 h of treatment. The inhibition was still observed after 48 h of treatment for peaks 5 and 13. The inhibitory effect of the KCN, alone or in combination with ASA disappeared after 48 h of treatment (Table 5). Several peaks, such as 1, 4, 6, 9 and 12 increased their secretion in response to the presence of ASA or ASA/KCN along the period of study (Tables 1, 3, 5, 7).
Under the treatment with orthovanadate the absence of peak 6 and the decrease of peak 13 were observed in comparison to the treatment with 100 µM ASA after 12 h (Table 1). The reduction of peaks 6 and 21, and the absence of the peak 18 were observed with the treatment of orthovanadate after 48 h (Table 5). The peaks 1, 4, 6, 8 and 21 were also reduced after 72 h of treatment (Table 7). Peak 4 incremented with the ASA treatment. Under the treatment with ASA/orthovanadate through the 72 h, the peaks 1, 4, 13 and 21 were reduced (Table 7). On the other hand, the peaks 2, 6, 8 and 9 were increased (Table 7).
The decrease of peaks 4, 6 and 14 were observed through the treatment with quinidine after 12 h (Table 2), as well as the absence of peak 1. After 48 h of treatment, peaks 4, 13 and 19 decreased (Table 6). From 24 to 72 h the absence of peaks 1, 2, 3 and the decrement of peak 21 was observed (Tables 4, 6, 8). When the hairy roots were treated with ASA/quinidine, some compounds such as, peaks 1, 2 and 3, were not detected (Tables 2, 4, 6, 8). However, other peaks, such as 4, 6, 11 and 20 increased after 72 h of treatment with ASA/quinidine (Table 8). It is important to indicate that most of these peaks increased with the treatment of 100 µM ASA in comparison with the respective control.
Several peaks decreased with the treatment of 100 µM glibenclamide. Peaks 1, 2, 6, 9, 13, 14, and 19 decreased after 12 h of treatment (Table 2). Peaks 2 and 21 were absence after 72 h of treatment (Table 8). On the other hand, several peaks increased with the treatment of ASA/glibenclamide, such as the case for peaks 2, 4, 6, 9 and 14 after 12 h of treatment (Tables 2, 4, 6, 8). Peaks 2, 3, 4 and 7, even after 72 h of treatment, are higher than the controls (Table 8).
The secretion of unknown compounds by C. roseus hairy roots did not change after the treatment with NO (data no showed). Indeed, we observed that the treatments with NO/KCN, NO/orthovanadate, NO/quinidine and NO/glibenclamide did not result in an inhibitory effect of the compounds secretion when comparing with the respectively controls.
HPLC analysis revealed clear differences in the pattern of secretion of the alkaloids from hairy roots treated with ASA and ABC transporters inhibitors (Tables 1-8) (2, 3, 4, 5, 6, 7). Using the principal components analysis (PC) we were able to determinate that the different treatments segregated from the control sample and they were clearly grouped along the first PC axis (Fig. 5). Interesting, at 72 h the treatments that include ASA make a clear group, except the treatment ASA/KCN. Moreover, the treatments with quinidine and glibenclamide were closed correlated with DMSO as control. On the other hand, a close correlation was observed between KCN and orthovanadate but not with the respective control (Fig. 5)
Discussion
In order to enhance and manipulate secondary metabolites mediated defense responses, it is necessary to understand the biosynthesis, regulation and transporting mechanism of these compounds. In the present study, we made an attempt to identify the different transporting systems to translocate the indole alkaloids by employing pharmacological approach with the help of inhibitors.
The secretion of secondary metabolites from the plant cells is often reported to be an energy-dependent transport [5, 25]. On the other hand, recent studies demonstrated that ABC transporters are involved in the transport of some secondary metabolites from plant root cells [13, 20, 26-28].
If the secretion of MIAs is mediated by ABC transporters, ATP must play a central role and inhibition of ATP production must modify the secretion process. We used potassium cyanide an inhibitor of ATP synthesis, [29], Sodium orthovanadate is an inhibitor of the membranal ATPases [30, 31] and also inhibits all kinds of ABC transporters [32, 33], quinidine and glibenclamide are potassium channel blockers [32]. Besides these, there are other type of sulfonylurea receptor inhibitors that are also able to inhibit the function of some ABC transporters [34].
In our study, the treatment of C. roseus hairy roots with KCN inhibited the accumulation of ATP, both in the presence or absence of an elicitor ASA (Fig. 1B) and it suggests that the inhibitor KCN clearly reducing the ATP levels in the cell. Shitan et al. [35] demonstrated that the addition of KCN inhibited the levels of berberine uptake by Cjmdr1-injected oocytes. Furthermore, Loyola-Vargas et al. [20] reported that KCN inhibited the roots-secretion of phytochemicals in Arabidopsis thaliana plants. Based on these observations, KCN is considered to be a good inhibitor and reduce ATP levels in the cell and it is worth to analyze the secretion levels MIA by supplementing KCN in hairy roots to determine the ABC transporters role in MIAs transport.
In A. thaliana P-type H+-ATPase isoforms found in membranes [36] and P-glycoproteins are strongly inhibited by vanadate [33, 37]. In our case, both potassium cyanide and sodium orthovanadate inhibited the alkaloid secretion of several peaks, specially numbers 2, 5 and 13 by C. roseus hairy roots (Tables 1, 3).
Orthovanadate is generally accepted as a strong inhibitor of ABC transporters [33, 37], and their inhibitory effect were observed on ajmalicine secretion after 48 h of treatment (Fig. 2B) and serpentine secretion after 24 h (Fig. 3B). In Lycopersicon peruvianum cell cultures, orthovanadate causes moderate alkalinization after it is added [38], suggesting a possible link between proton fluxes across the plasma membrane and the secretion of compounds into the rhizosphere. However, alkalinization did not occur, since the pH value of the medium, during the culture period varied from 5.75 to 6.34, for all the treatments including the controls. These data suggest that the secretion process is ATP-dependent and that either a primary or secondary active transporter could be involved in the secretion of phytochemicals into the culture medium by C. roseus hairy roots.
When C. roseus hairy roots were cultured in MS medium we found that the conductivity of the medium was between 2.1 and 3 mSi for the treatments. These data suggest that it is more probable that the secretion of phytochemicals occurs through a primary transporter. Nevertheless, we cannot discard the possibility that in the microenvironment of the apoplast, around the cell wall, the concentration of protons is enough to drive the efflux of phytochemicals. Another possibility is that the secreted phytochemicals are stored in the vacuole; if this is the case, it is possible that the secretion of the compounds is mediated by active secondary transport systems that require the V-ATPase and vacuolar pyrophosphatase for maintenance of a proton gradient across the tonoplast.
Another two compounds which inhibit the activity of various transporters were examined by addition to the medium at the concentration of 100 mM of each inhibitor. Quinidine and glibenclamide are inhibitors of channel blockers and of ABC transporters [39, 40] that function as a drug efflux pump in human cancer cells [41]. In plants, it is well documented the role of ABC transporters in translocating the secondary metabolites by using inhibitors. For instance, berberine uptake by a vacuolar P-glycoprotein is inhibited by nifedipine and quinidine in a dose-dependent manner [42]. Verapamil, nifedipine and glibenclamide also inhibit berberine uptake in Cjmdr1-injected oocytes [35]. It has also been shown that an ABC-type efflux-transporter is functioning in Thalictrum minus suspension cultures [43]. Geisler et al. [44] by using MDR/PGP inhibitors like cyclosporin A and verapamil inhibited the efflux of auxin in PGP 1 transformed yeast.
As shown in tables 1-8 (2, 3, 4, 5, 6, 7), and figures 2C, 3C and 3D the secretion of some unidentified phytochemicals as well as ajmalicine and serpentine produced by C. roseus hairy roots was inhibited by these compounds, suggesting that a primary transporter might be involved in their exudation into the medium. Only the secretion of a few compounds is inhibited by each inhibitor, suggesting that the inhibition process is very selective and that different transporters are involved in the secretion of the different secondary metabolites by the hairy roots. However, in some cases unrelated compounds can be moved by the same transporters, like yeast pdr5p which is able to transport flavonoids, indole alkaloids and taxol [45].
In our research the secretion of MIAs by hairy roots treated with the different inhibitors in presence of ASA did not followed the same pattern for all the alkaloids, suggesting the presence of more than one MIAs transport mechanism. For instance, ajmalicine was the most abundant alkaloid secreted by the treatment with ASA/KCN after 72 h (Fig. 2A). However, at the same time the treatment with ASA/orthovanadate significantly decreased the secretion of ajmalicine when compared with the treatment with ASA (Fig. 2B). A similar effect was observed for the secretion of serpentine with the treatments with ASA/KCN and ASA/quinidine after 72 h of treatment (Figs. 3A and 3C).
HPLC analysis reveals the existence of 22 main peaks present in the C. roseus hairy roots exudates. The peaks were enumerated from 1 to 22 following the elution time. The peaks 3, 11 and 12 corresponded to serpentine, ajmalicine and catharanthine respectively. Among the unknown peaks, the secretion of twelve of them (1, 2, 4, 6, 8, 9, 13, 14, 15, 18, 19 and 21) were inhibited or reduced when the hairy roots was treated with KCN, orthovanadate, quinidine or glibenclamide (Tables 1, 2, 3, 4, 5, 6, 7 and 8). However, in many of the cases the treatments with KCN, orthovanadate, quinidine or glibenclamide in presence of ASA, induced again the secretion of these compounds (Tables 1, 2, 3, 4, 5, 6, 7 and 8), suggesting that the increase in the amount of alkaloids produced by the ASA induction is higher than inhibition of the transport produced by the inhibitor. Also could be possible that the ASA induced the presence or additional transporters, which in turn can increase the secretion of the alkaloids. In transgenic plant cell suspension cultures of N. tabacum, carrying PRD5 genes from yeast, it as has been shown that those genes can be used to stimulate the secretion of secondary metabolites [46].
The increase in secretion of some peaks (Tables 1-8) (2, 3, 4, 5, 6, 7) observed at 100 µM quinidine could be the result of a plasmatic membrane leak because of the strong inhibition of the transport system; however, this explanation appears improbable since there is a clear inhibition of several other peaks. It is possible that the increase in secretion of these peaks may be an indirect effect of the inhibitor since some transporters, such as P-glycoprotein and members of the multidrug-resistance-related protein (MRP) subfamily in animal cells are able to efflux anticancer drugs from the cytosol [47]).
Glibenclamide inhibits the potassium channels and some ATP transporters in animal cells [34]. When added to hairy roots cultures inhibited the secretion of several peaks, including serpentine (Fig. 3; Tables 1-8) (2, 3, 4, 5, 6, 7). Recently it has been shown that a Crmdr1 is constitutively expressed in the root of C. roseus plants and that may be involved in the transport and accumulation of secondary metabolites [48].
On the other hand, glibenclamide, in the presence of NaCl, inhibits the growth of the roots of the mutant Atmrp5-2 grown in NaCl alone [49]. This inhibition is reversed by diazoxide, a known K+- channel opener that reverses the inhibitory effects of sulfonylureas in animal cells. However, it cannot discard the possibility of an indirect effect of this compound with MATE transporters or the possible indirect effect on vacuolar, pH-dependent transport [50].
Conclusions
Taken together, the data presented here provides evidence that ABC transporters could be involved in the secretion of MIAs. Understanding the biosynthetic pathway of these compounds is important, indeed the understanding of the transporting mechanisms of these compounds would be a novel area to engineer the plants to enhance the secretion of these compounds to increase the value of secondary metabolites for plant to defend biotic and abiotic stresses that lead to plant health and production [51].
Experimental section
Plant material and growth conditions
Catharanthus roseus hairy roots were obtained through genetic transformation of roots with Agrobacterium rhizogenes strain 1855 bearing plasmid pBI 121.1 [52]. The hairy roots were maintained by sub-culturing every 15 days using half-strength Gamborg B5 medium [53] supplemented with 3% (w/v) of sucrose. The cultures were kept on orbital shakers at 100 rpm in the dark at 25 ± 2 ºC.
Inhibition assays
Fourteen-day-old hairy roots were washed with water twice and transferred in to liquid Gamborg B5 media supplemented with 3% (w/v) of sucrose, elicitors and/or inhibitors (100 µM potassium cyanide, KCN; 100 µM sodium orthovanadate, Na3VO4; 100 µM quinidine or 100 µM glibenclamide). KCN and Na3VO4 were dissolved in water while quinidine and glibenclamide were dissolved in DMSO. The elicitors used in this study were acetylsalicylic acid (ASA; 100 µM) and sodium nitroprusside (Na2[Fe(CN)5NO]; 10 µM) as nitric oxide (NO) donor. Both elicitors were dissolved in water. The experiment was conducted with 13 treatments in different combinations of inhibitors and elicitors and their respective controls. The hairy roots were exposed to ASA and collected the tissues at 12, 24, 48 and 72 hours and to NO for only 12 hours. In each sampling-time, the plant material was weighed and alkaloids were extracted from both the culture media and the hairy roots.
Fresh (FW) and dry weight (DW) determination
After 12, 24, 48 and 72 h of elicitation with 10, 100 and 250 µM of ASA, hairy roots were collected and weighed for FW determination. For DW determination, the roots were frozen at -80°C and freeze-dried. After total elimination of water was achieved, the lyophilized roots were weighted. Each sample was done by triplicate. The experiment was repeated three times with triplicate.
ATP determination by high performance liquid chromatography (HPLC)
ATP was extracted from the mitochondrial sample as previously reported by Yang et al. [54]. The HPLC method reported by Liu et al. [55] was followed. Briefly, sample from isolated mitochondria were chromatographed by gradient elution on a 4.6 mm x 150 mm reverse phase, Zorbax Eclipse XDB, 5 µm particle size C18 column (Agilent Technology). The chromatographic system (Agilent series 1200) consists of quaternary G1311A pumps connected to a G1329A automatic sample injector. The injected samples (20 µL) were detected at 254 nm with Gold 168 diode array detector G1315B (Agilent technology). The mobile phase A consisted 60 mM K2HPO4 and 40 mM KH2PO4 dissolved in HPLC quality water and adjusted to pH 7.0 with 100 mM KOH, while mobile phase B consisted of 100% acetonitrile. HPLC separation was achieved using continuous gradient elution. ATP in the samples were identified by comparison with the retention time of the standards, while the concentrations of ATP were determined using the external standard method. Data were expressed as means of three replicate determinations.
Extraction of alkaloids from culture medium
To examine the secreted phytochemicals from C. roseus hairy roots, liquid media samples from in vitro-grown Catharanthus hairy roots were collected (final volume of 100 mL), filtered through a nylon syringe filter of pore size 0.45 µm (Life Sciences Cat. PN 4612 or Nalgene cat. 195-2520) to remove any cellular debris, and concentrated by freeze-drying (Labconco) to remove water. The concentrate was dissolved in 5 mL 2.5% (v/v) sulphuric acid and extracted as described by Monforte et al., [56]. The final concentrate was dissolved in 500 µL of absolute methanol (Fisher Scientific Co.) and analyzed by HPLC. The same procedure was followed for each treatment.
HPLC analysis of alkaloids from the exudates
Compounds from roots and media were chromatographed by gradient elution on a 4.6 mm x 150 mm reverse phase, Zorbax Eclipse XDB, 5-µm particle size C8 column (Agilent technology). The chromatographic system (Agilent series 1100) consists of two G1312A pumps connected to a G1328A manual sample injector. The injected samples (20 µL) were detected at 280 nm with a variable UV-vis detector G1365B (Agilent technology). The mobile phase consisted of acetonitrile: 10 mM (NH4)2HPO4 (43:57) a flow rate of 1.5 mL min-1. The solution was filtered through a 0.22 µm nylon filter and degassed under vacuum.
Retention times and peak heights of commercially purchased ajmaline, serpentine, vincamine, vindoline, ajmalicine and catharanthine (Sigma Chemical Co.), were run in HPLC to determine the possible presence and concentration of compounds in the root exudates and tissues.
Statistical Analysis
Each experiment was conducted with triplicate. The statistical analysis was performed by one-way ANOVA analysis, taking P ≤ 0.05 and P ≤ 0.01 (Tukey's test) as significant and highly significant, respectively. Principal components analysis (PCA) was performed using the average of the peak areas of the chromatogram of each treatment (see Figs. 4 and 5) to identify the most important variables in the secretion of compounds. Matrix was constructed with Pearson's correlation coefficients [57], using the program MVSP Version 3.13q Copyright© 1985-2008 Kovach Computing Service (http://www.kovcomp.com). The first three axes account for >69% of total variation, giving a clear idea of the structure underlying the quantitative variables analyzed.
Acknowledgements
We acknowledge the research funds provided by CONACYT (Grant No. 157014).
References
1. Zhu, X.; Zeng, X.; Sun, C.; Chen, S. Front. Med. 2014. 8, 285-293. [ Links ]
2. Oudin, A.; Courtois, M.; Rideau, M.; Clastre, M. Phytochem. Rev. 2007, 6, 259-276. [ Links ]
3. Facchini, P. J.; De Luca, V. Plant. J. 2008, 54, 763-784. [ Links ]
4. Dugé de Bernonville, T.; Clastre, M.; Besseau, S.; Oudin, A.; Burlat, V.; Glevarec, G.; Lanoue, A.; Papon, N.; Giglioli-Guivarc'h, N.; St-Pierre, B.; Courdavault, V. Phytochemistry. 2015. 113, 9-23. [ Links ]
5. Deus-Neumann, B.; Zenk, M. H. Planta. 1984, 162, 250-260. [ Links ]
6. McCaskil, D. G.; Martin, D. L.; Scott, A. I. Plant Physiol. 1988, 87, 402-408 [ Links ]
7. Neumann, D.; Krauss, G.; Hieke, M.; Groger, D. Planta Med. 1983, 48, 20-23. [ Links ]
8. Carqueijeiro, I.; Noronha, H.; Duarte, P.; Geros, H. V.; Sottomayor, M. Plant Physiol. 2013, 162, 1486-1496. [ Links ]
9. Pomahacova, B.; Dusek, J.; Duskova, J.; Yazaki, K.; Roytrakul, S.; Verpoorte, R. J. Plant Physiol. 2009, 166, 1405-1413. [ Links ]
10. El-Guizani, T.; Guibert, C.; Triki, S.; St-Pierre, B.; Ducos, E. J. Genet. 2014, 93, 21-33. [ Links ]
11. Loyola-Vargas, V. M.; Galaz-Avalos, R. M.; Rodríguez-Ku, J. R. Phytochem. Rev. 2007, 6, 307-339. [ Links ]
12. Roepke, J.; Salim, V.; Wu, M.; Thamm, A. M. K.; Murata, J.; Ploss, K.; Boland, W.; De Luca, V. Proc. Natl. Acad. Sci. (USA). 2010, 107, 15287-15292. [ Links ]
13. Yu, F.; De Luca, V. Proc. Natl. Acad. Sci. (USA). 2013, 110, 15830-15835. [ Links ]
14. Vázquez-Flota, F.; Moreno-Valenzuela, O. A.; Miranda-Ham, M. d. L.; Coello-Coello, J.; Loyola-Vargas, V. M. Plant Cell Tiss. Org. Cult. 1994, 38, 273-279. [ Links ]
15. Ruíz-May, E.; Galaz-Avalos, R. M.; Loyola-Vargas, V. M. Mol. Biotechnol. 2009, 41, 278-285. [ Links ]
16. Lee-Parsons, C. W. T.; Ertük, S. Plant Cell Rep. 2005, 24, 677-682. [ Links ]
17. Godoy-Hernández, G.; Loyola-Vargas, V. M. Plant Cell Rep. 1997, 16, 287-290. [ Links ]
18. Loyola-Vargas, V. M.; Ruíz-May, E.; Galaz-Avalos, R. M.; De-la-Peña, C. Plant Signal. Behav. 2012, 7, 611-614. [ Links ]
19. Eichhorn, H.; Klinghammer, M.; Becht, P.; Tenhaken, R. J. Exp. Bot. 2006, 57, 2193-2201. [ Links ]
20. Loyola-Vargas, V. M.; Broeckling, C. D.; Badri, D. V.; Vivanco, J. M. Planta. 2007, 225, 301-310. [ Links ]
21. Sugiyama, A.; Shitan, N.; Yazaki, K. Plant Physiol. 2007, 144, 2000-2008. [ Links ]
22. Badri, D. V.; Loyola-Vargas, V. M.; Du, J.; Stermitz, F. R.; Broeckling, C. D.; Iglesias-Andreu, L. G.; Vivanco, J. M. New Phytol. 2008, 179, 209-223. [ Links ]
23. Baetz, U.; Martinoia, E. Trends Plant Sci. 2014, 19, 90-98. [ Links ]
24. De-la-Peña, C.; Loyola-Vargas, V. M. Plant Physiol. 2014, 166, 701-719. [ Links ]
25. Yamamoto, H.; Suzuki, M.; Suga, Y.; Fukui, H.; Tabata, M. Plant Cell Rep. 1987, 6, 356-359. [ Links ]
26. Yazaki, K. FEBS Lett. 2006, 580, 1183-1191. [ Links ]
27. Badri, D. V.; Loyola-Vargas, V. M.; Broeckling, C. D.; De-la-Peña, C.; Jasinski, M.; Santelia, D.; Martinoia, E.; Sumner, L. W.; Banta, L. M.; Stermitz, F. R.; Vivanco, J. M. Plant Physiol. 2008, 146, 762-771. [ Links ]
28. Yu, F.; De Luca, V. Transport of monoterpenoid indole alkaloids in Catharanthus roseus, Springer International Publishing, Plant ABC Transporters (Geisler, M.; ed.), Switzerland. 2014, 63-75. [ Links ]
29. Lew, R. R.; Spanswick, R. M. Plant Physiol. 1984, 75, 1-6. [ Links ]
30. Cantley, L. C., Jr.; Cantley, L. G.; Josephson, L. J. Biol. Chem. 1978, 253, 7361-7368. [ Links ]
31. Willsky, G. R.; White, D. A.; McCabe, B. C. J. Biol. Chem. 1984, 259, 13273-13281. [ Links ]
32. Horio, M.; Gottesman, M. M.; Pastan, I. Proc. Natl. Acad. Sci. (USA). 1988, 85, 3580-3584. [ Links ]
33. Urbatsch, I. L.; Sankaran, B.; Weber, J.; Senior, A. E. J. Biol. Chem. 1995, 270, 19383-19390. [ Links ]
34. Golstein, P. E.; Boom, A.; van Geffel, J.; Jacobs, P.; Masereel, B.; Beauwens, R. Pflüg. Arch. Eur. J. Phy. 1999, 437, 652-660. [ Links ]
35. Shitan, N.; Bazin, I.; Dan, K.; Obata, K.; Kigawa, K.; Ueda, K.; Sato, F.; Forestier, C.; Yazaki, K. Proc. Natl. Acad. Sci. (USA). 2003, 100, 751-756. [ Links ]
36. Palmgren, M. G.; Christensen, G. J. Biol. Chem. 1994, 269, 3027-3033. [ Links ]
37. Taguchi, Y.; Yoshida, A.; Takada, Y.; Komano, T.; Ueda, K. FEBS Lett. 1997, 401, 11-14. [ Links ]
38. Schaller, A.; Oecking, C. Plant Cell. 1999, 11, 263-272. [ Links ]
39. Wigler, P. W. J. Bioenerg. Biomembr. 1996, 28, 279-284. [ Links ]
40. Boumendjel, A.; Baubichon-Cortay, H.; Trompier, D.; Perrotton, T.; Di Pietro, A. Med. Res. Rev. 2005, 25, 453-472. [ Links ]
41. Ueda, K.; Cardarelli, C.; Gottesman, M. M.; Pastan, I. Proc. Natl. Acad. Sci. (USA). 1987, 84, 3004-3008. [ Links ]
42. Sakai, K.; Shitan, N.; Sato, F.; Ueda, K.; Yazaki, K. J. Exp. Bot. 2002, 53, 1879-1886. [ Links ]
43. Terasaka, K.; Shitan, N.; Sato, F.; Maniwa, F.; Ueda, K.; Yazaki, K. Plant Cell Physiol. 2003, 44, 198-200. [ Links ]
44. Geisler, M.; Blakeslee, J. J.; Bouchard, R.; Lee, O. R.; Vincenzetti, V.; Bandyopadhyay, A.; Titapiwatanakun, B.; Peer, W. A.; Bailly, A.; Richards, E. L.; Ejendal, K. F. K.; Smith, A. P.; Baroux, C.; Grossniklaus, U.; Muller, A.; Hrycyna, C. A.; Dudler, R.; Murphy, A. S.; Martinoia, E. Plant J. 2005, 44, 179-194. [ Links ]
45. Kolaczkowski, M.; van der Rest, M.; Cybularz-Kolaczkowska, A.; Soumillion, J. P.; Konings, W. N.; Goffeau, A. J. Biol. Chem. 1996, 271, 31543-31548. [ Links ]
46. Goossens, A.; Hakkinen, S. T.; Laakso, I.; Oksman-Caldentey, K. M.; Inze, D. Plant Physiol. 2003, 131, 1161-1164. [ Links ]
47. Glavinas, H.; Krajesi, P.; Cserepes, J.; Sarkadi, B. Curr. Drug Del. 2004, 1, 27-42. [ Links ]
48. Jin, H.; Liu, D.; Zuo, K.; Gong, Y.; Miao, Z.; Chen, Y.; Ren, W.; Sun, X.; Tang, K. DNA Sequence. 2007, 18, 316-325. [ Links ]
49. Lee, E. K.; Kwon, M.; Ko, J.-H.; Yi, H.; Hwang, M. G.; Chang, S.; Cho, M. H. Plant Physiol. 2004, 134, 528-538. [ Links ]
50. Frangne, N.; Eggmann, T.; Koblischke, C.; Weissenbock, G.; Martinoia, E.; Klein, M. Plant Physiol. 2002, 128, 726-733. [ Links ]
51. Yazaki, K. Curr. Opi. Plant Biol. 2005, 8, 301-307. [ Links ]
52. Ciau-Uitz, R.; Miranda-Ham, M. d. L.; Coello-Coello, J.; Chí, B.; Pacheco, L. M.; Loyola-Vargas, V. M. In Vitro Cell. Dev. Biol. -Plant. 1994, 30, 84-88. [ Links ]
53. Gamborg, O. L.; Miller, R. A.; Ojima, K. Exp. Cell Res., 1968, 50, 151-158. [ Links ]
54. Yang, N. C.; Ho, W. M.; Chen, Y. H.; Hu, M. L. Anal. Biochem. 2002, 306, 323-327. [ Links ]
55. Liu, H.; Jiang, Y. M.; Luo, Y. B.; Jiang, W. B. Food Technol. Biotech. 2006, 44, 531-534. [ Links ]
56. Monforte-González, M.; Ayora-Talavera, T.; Maldonado-Mendoza, I. E.; Loyola-Vargas, V. M. Phytochem. Anal. 1992, 3, 117-121. [ Links ]
57. Sneath, P. H. A.; Sokal, R. R. Numerical taxonomy: the principles and practice of numerical classification, 1973. [ Links ]














