Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Journal of the Mexican Chemical Society
versão impressa ISSN 1870-249X
J. Mex. Chem. Soc vol.59 no.2 Ciudad de México Abr./Jun. 2015
Articles
Synthesis and Characterization of K2Ln2/3Ta2O7·nH2O (Ln= La, Pr, Nd), Layered Tantalates Photocatalysts for Water Splitting
Hoover Valencia-Sanchez,1,5* Heriberto Pfeiffer,2 Dwight Acosta,3 Alicia Negron-Mendoza4 and Gustavo Tavizon1
1 Facultad de Química. Universidad Nacional Autónoma de México. Ciudad Universitaria, C.P. 04510, México D.F.
2 Instituto de Investigaciones en Materiales. Universidad Nacional Autónoma de México. Ciudad Universitaria, C.P. 04510, México D.F.
3 Instituto de Física. Universidad Nacional Autónoma de México. Ciudad Universitaria, C.P. 04510, México D.F.
4 Instituto de Ciencias Nucleares; Universidad Nacional Autónoma de México. Ciudad Universitaria, C.P. 04510, México D.F.
5 Permanent address: Escuela de Química, Universidad Tecnológica de Pereira, Carrera 27 10-02 Los Alamos, C. P. 660003, Pereira-Colombia, Tel. (+57)63137242, hvalencia@utp.edu.co
Received July 7th, 2014.
Accepted January 27th, 2015
Abstract
Three compounds of the K2Ln2/3Ta2O7 (Ln=La, Nd, Pr) cation-deficient Ruddlesden-Popper series were prepared by the Pechini (polymeric complex) method. The crystal structures of the hydrated form of these compounds were determined by Rietveld analysis of the X-ray powder diffraction data and High Resolution Transmission Electron Microscopy (HRTEM). The samples were also analyzed to determine specific area (BET), degree of hydration (TGA), and photocatalytic activity for hydrogen evolution from water and aqueous methanol solution.
Key words: Photocatalyst, Water Splitting, Tantalates, Ruddlesden-Popper, Hydrogen production.
Resumen
Tres compuestos del tipo K2Ln2/3Ta2O7 (Ln=La, Nd, Pr) de la serie de Ruddlesden-Popper deficientes de cationes fueron preparados por el método de Pechini (complejo polimerizable). Las estructuras cristalinas de los compuestos hidratados fueron determinadas por análisis de Rietveld de los patrones de difracción de rayos X y mediante microscopia electrónica de alta resolución (HRTEM). Las muestras también fueron analizadas para determinar su área superficial (BET); grado de hidratación (TGA), y actividad fotocatalítica en la producción de hidrógeno a partir de agua y de soluciones metanol/agua.
Palabras Clave: Fotocatalizadores, Disociación del agua, Tantalatos, Ruddlesden-Popper, Producción de Hidrógeno.
Introduction
Tantalum-oxide based compounds crystallizing in the Ruddlesden-Popper (RP) structure [1] have recently attracted much attention due to their ion-exchange[2]–[5] (96 and intercalation [6]–[8] properties and as photocatalysts for hydrogen generation from water splitting [9]–[11]. Additionally, high proton conductivity has been recently reported [12] in randomly oriented grains of H2SrTa2O7 and SrTa2O6. In their anhydrous form, the K2[Ln3/2Ta2O7] RP tantalates correspond to the formula A'2[An-1BnO3n+1], where A' is often an alkali metal, A is a rare earth metal, B is a transition metal, and n defines the number of BO6 octahedra forming perovskite layers[13]. In this structure there are two A' interlayer cations per formula unit interconnecting the n perovskite sheets. Most of the compounds of the RP series with B=Ti and/or Ta crystallize in the space group (SG) I4/mmm (No. 139), which consists of perovskite blocks separated by rock-salt type A'O blocks. This structure produces a staggered arrangement in which two contiguous perovskite blocks are shifted by (a+b)/2 in the crystal cell. During intercalation of water into the RP structure, the water molecules form new layers inside the rock-salt type block[14], and a structural change from the I4/mmm to the P4/mmm (No. 123) SG occurs [15]. As reported for several layered tantalates with photocatalytic water splitting activity [9],[10],[16],[17], the interlayer hydration plays a key role because the intercalated water molecules are accessible to the active sites responsible for the photocatalytic reaction. The number of publications on the photocatalytic activity of layered tantalates has increased in recent years, such as Kudo et al. [18] reported. These new semiconductors are laminar compounds exhibiting a layered structure in which perovskite blocks consisting of TaO6 octahedra are thought to be responsible of such photoactivity. One of them, the NaTaO3 has the highest quantum yield in the photocatalytic reaction that splits water into H2 and O2. This compound was studied using different lanthanide doping such as: La, Pr, Nd, Sm, Gd, Tb and Dy[19]. NaTaO3 presents a quantum yield of 56% at 270 nm when it is doped with lanthanum and Ni-loading (NaTaO3:La (2%) NiO (0,2 % w))[20],[21]. On the other hand, the undoped compound, NaTaO3:NiO (0,05 %w), presented a yield of 28% at 270 nm (surface specific area of 0,52 m2/g) as reported Kato et al. [22]. The significant decrease in particle size and a topography in which terraces predominate separating active sites for oxidation and reduction are thought to be the main responsible factors of such phenomenon[23].
With the aim of investigating the synthetic route and the main crystal features of hydrated layered tantalates and to explore the effect of the partially filled 4f shell cations (Pr, Nd) on photocatalytic activity for hydrogen evolution of the RP tantalates, a study of the K2Ln3/2Ta2O7 (Ln=La, Pr, Nd) series of compounds is presented. These compounds were synthesized via the Pechini method (PC) that results in good crystallinity of the samples and higher or equal specific areas than the synthesized by the solid-state reaction method. These characteristics are important in the heterogeneous photocatalytic process of these systems. The compounds synthesized in this work are lanthanide-deficient (1/3 per formula unit) and, as reported for the RbLnTa2O7 tantalate[24], the role played by these lanthanides in the neighborhood of the TaO6 octahedra cannot be considered as that of “spectator” in the photocatalytic activity behavior of these layered perovskite tantalates.
Results and Discussion
XRD patterns of the hydrated phase (HP) of K2La2/3Ta2O7 (LaN2), K2Pr2/3Ta2O7 (PrN2), and K2Nd2/3Ta2O7 (NdN2) are shown in Fig. 1. In the Table 1, a list of reflections that correspond to diffraction pattern of the Fig 1 is presented. The reflections marked with (*) in Table 1, contribute to the total intensity and the observed reflection corresponds to the sum of several reflections. For example, the first reflection should be assigned to the 003, 010 and 011 contributions. The second one results from the 013, 110, 111 and 014 reflections. The formation of the PR phases were also supported by HRTEM (see below). Likewise, the wide character of the main reflections observed in these patterns must be associated with the small size of crystallites obtained by the Pechini method of synthesis. In this synthetic route, an important element to consider refers to the calcination temperature because once this exceeds 900°C, undesired tungsten-bronze type compounds appear in the XRD patterns[25]. Several authors have referred to the appearance of tungsten bronzes, obtained by the solid-state reaction method[26]–[28].

To determine the number of hydrating water molecules in samples, dynamic thermogravimetric analysis (TGA) was performed and the compounds were heated under airflow from 25°C to 1000 °C (10 °C/min). To consider only the water molecules intercalated into the crystal structure, without taken into account the water molecules on the crystal surface, the weight-loss was considered from 100°C. According to these thermogravimetric analyses, the number of hydration water molecules for LaN2, PrN2 and NdN2 is 2.5, 1.8 and 0.83, respectively. A plot of this thermal behavior is presented in Fig. 2. As observed in this plot, the number of intercalated water molecules in these layered structure compounds is different for each lanthanide, decreasing in the order LaN2>PrN2>NdN2.

As previously reported for alkaline laminar tantalates (except Li[10],[16],[17],[27], Cs and Rb[29]), these compounds exhibit spontaneous water intercalation when exposed to air under room temperature[27],[28],[30]. For the anhydrous phase of K2La2/3Ta2O7, Crosnier-Lopez et al [27] determined an I4/mmm space group with α = 3.9679Å and c = 22.0807Å crystal cell parameters. Water intercalation of this compound is extremely rapid and one structural transition was observed from the body centered (I4/mmm) to a primitive lattice (P4/mmm) in the hydrate form of this compound, K2La2/3Ta2O7·2H2O. For this compound the cell parameters are α = 3.9427Å and c = 12.887Å[27]. As occurs in other laminar compounds of the Ruddlesden-Popper series as K2Nd2Ti3O10, water intercalation produces a change in the symmetry due to an (α+b)/2 sliding of the central slab relative to the adjacent slabs [31]. The SG for all the LnN2 (Ln = La, Pr and Nd) hydrated phases is P4/mmm and the diffraction patterns are presented in Fig. 1.
Crystal structure of K2La2/3Ta2O7 has been previously reported by Crosnier-Lopez et al[27] through Rietveld refinements of XRD data. In the present work, Rietveld refinement of the hydrated form of this phase is presented, and this study has been extended to include the Pr and Nd cations in the crystal unit cell (see Fig. 3). For the Rietveld analysis the P4/mmm SG was attempted[4], and the water molecule position was the 2e Wyckoff site (0, 1/2, 1/2; 1/2, 0, 1/2); however, its occupancy was not refined and the different water stoichiometries of the compounds correspond to those estimated from thermal analysis. However, the values of the isotropic thermal parameters for Ow (oxygen atom of the water molecule) are acceptable for LaN2, PrN2 and NdN2, when considering the interlayer distance in which those molecules are located. Site-occupancy refinements of Ta and O were also omitted because of the low volatility (and very low solubility in water) of the metal-oxide, and stability of the oxidation state of Ta. Considering the αb plane as that formed by the O(2) at the 4i Wyckoff position, Ta rises from this plane and the Ta-O(2)-Ta angle is 163.7° (LaN2); 163.56°(PrN2) and 164.66°(NdN2). In the LnN2 series, the Ta-(O2)-Ta angle remains almost constant, independent of the H2O/Ta ratio. For the cell parameters of the rare earth tantalates (see Table 1) there is no correlation between the lanthanide ionic radii (1.36, 1.31 and 1.27Å for La3+, Pr3+ and Nd3+, respectively, all them in coordination 12[32]) and the values of the α and c parameters with the water content in the crystal structure of the HPs. Comparing the water content to LnN2 compounds, the larger water content corresponds to the shorter distance between the slice of the TaO6 octahedra (see Fig. 3). Hydrated systems that exhibit the configuration of adjacent perovskite blocks with alkaline or alkaline-earth metals in the rock salt block have been reviewed by Lehtimäki et al[14]. According to this study, the main factors affecting the water intercalation are: the size of cations in the rock-salt block, the oxygen content of compounds, and the valence of the transition metal in the structure.

(Table 2)
An additional aspect of the LnN2 crystal structure, which is defective in 1/3 of the Ln3+ ion per unit formula, is that we have not observed ordering of vacancies that could be shown as additional reflections in the XRD pattern. This is probably due to the particle small size of the hydrated samples and the width of reflections. For K2La2/3Ta2O7, Crosnier-Lopez et al[27] observed by XRD and HRTEM, that the atomic vacancies are restricted to 9-coordinated K sites. As observed the diffraction patterns of the HP of LnN2 (see Fig. 1), all of them exhibit the characteristic broad reflections associated to the presence of very small crystallites in samples. Using the Scherrer equation to evaluate the average size of the crystallite, we obtain 4 nm. By Scanning Electron Microscopy (not shown), the average size of particles is about 50 nm.
(Fig. 4)
The HRTEM images of LaN2 (Fig. 5) reveal the good crystallinity of the tetragonal phase, this confirms the fact that a single phase can be obtained by the synthetic route of this work. Most of the HRTEM micrographs exhibit patterns corresponding to the (110), (100), (111), (010), (011), (014) and (013) crystallographic planes, with interplanar spacings of approximately 2.82, 3.69, 2.68, 4.12, 3.67, 2.64 and 2.99Å, respectively. The incidence of these planes reveals a predominant preferential orientation[27] of crystals in the LaN2 phase. These orientations agree well with the intense reflections observed in the DRX pattern. By HRTEM, the existence of an infrequent plane was also found, the (002) plane with 6.12Å (see Fig. 5a zone I, Fig. 5e zone I). Based on the goodness-of-fit parameters (Rp, Rwp, Rexp and χ2) of the structural refinements and the HRTEM images, the synthesis of the HPs of K2Ln2/3Ta2O7 was confirmed.
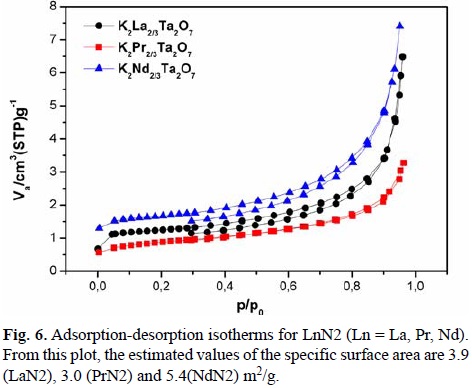
The adsorption-desorption isotherms
To determine the specific surface area of the HP of LnN2, the BET method was used and the graphical results are presented in the Fig. 6. Adsorption isotherms of K2Ln2/3Ta2O7 can be associated to the type II of the IUPAC classification, which represent an unrestricted monolayer-multilayer adsorption[33]. For most of the compounds at low P/Po (~0.25-0.35) ratio, the stage of complete monolayer coverage can be observed (see Fig. 6). The most relevant feature of this isotherm is that they did not exhibit any limiting adsorption at high P/Po ratio. This behavior can be caused by the existence of non-rigid aggregates of plate-like particles of slit-shaped pores[34]. The BET surface areas of the LnN2 compounds of the present work are 3.0 m2/g, 3.9 m2/g, and 5.4 m2/g for the HPs of K2Pr2/3Ta2O7, K2La2/3Ta2O7, and K2Nd2/3Ta2O7, respectively. These values are in the order reported for K2La2/3Ta2O7 (3.7 m2/g), obtained by the solid state reaction method[9]. This behavior can be directly associated to the kind of cation (alkaline or alkaline-earth metals).
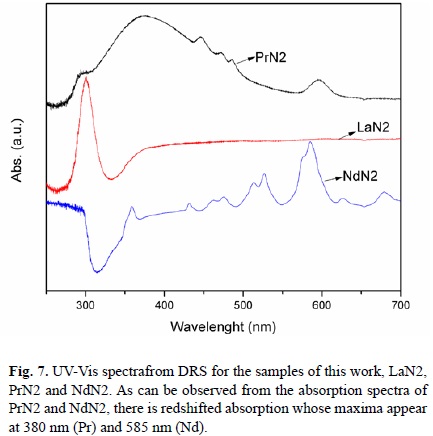
Absorbance spectra
To obtain the absorbance spectra of the K2Ln2/3Ta2O7 compounds, these were scanned in the wavelength range from 250 nm to 700 nm, using the DRS technique[35],[36]. As observed in the absorption spectra of the Fig. 7, for PrN2 and NdN2, redshifted absorption appear with maxima at 380 nm (Pr) and 585 nm (Nd). The band gaps values were calculated with the Kubelka-Munk function, and the values obtained are: 3.70 eV, 3.6 eV, and 3.5 eV for LaN2, PrN2, and NdN2, respectively. These absorption signals are associated with internal transitions in the localized 4f states[37], even though they are expected to be sharp peaks. This graph (Fig 7), and electronic structure calculations, support [38] the incomplete localization of the Ln (Pr and Nd) 4f electrons and thus its participation in the chemical bonding via orbital hybridization in the Ln-O-Ta system, while the interlayer cations (K) have a negligible effect on the electronic structure of layered Ln-tantalates. As expected from previous considerations, in the MLnTa2O7 (M=Cs, Rb, Na and H; Ln=La, Pr, Nd and Sm) series, a Dion-Jacobson type system, the experimental activity in water photolysis decreases in the sequence Nd>Sm>La>Pr; the low activity in the Pr system is explained by their acting as a trapping center of electronic holes[29].
Photocatalytic activity
The photocatalytic activity of the LnN2 compounds is presented in the graph (Fig. 8). The photocatalytic reaction was carried out in an inner irradiation quartz cell. The catalyst was dispersed in aqueous methanol solution (14% vol/vol), using deionized water, then it was irradiated by a high pressure Hg lamp (400W). Amounts of evolved hydrogen gas were analyzed by gas chromatography. The highest activity was achieved when the catalysts were used in aqueous methanol mixture (see Table 3), except to PrN2. On the other hand, as the larger surface area corresponds to NdN2; it is considered that the smaller particle size increases the activity for water splitting. Comparing the most active powder (NdN2), the activity was higher by twice than that when LaN2 and PrN2 were used in an aqueous methanol solution (97.5 μmol H2/g.h). When the reaction was tried in pure water (see Table 4), the NdN2 compound also had higher activity than PrN2 and LaN2; although the NdN2 and LaN2 activity decreased in pure water, while that of PrN2 remained low. Therefore, the high photocatalytic activity of the NdN2 sample should be associated with the high specific surface area of this sample. In an analogous system, RbLnTa2O7 (Ln = La, Pr, Nd, Sm), Machida et al. [39] showed that the Nd system also exhibited the highest activity. The explanation of this activity is associated to the electronic structure change that comes out from the unfilled 4f levels of the lanthanide. The Ln-O-Ta hybridization affects the position of both; the conduction band and the valence band edges, as well as their density of states (DOS)[38]. Electronic structure calculations show[37]–[39] that the unoccupied 4f (Ln=La) levels lay at the bottom of the conduction band, whereas the occupied 4f levels become lowered as the number of 4f electrons increases. Finally, these levels overlap with O-2p band for Ln = Nd and Sm [37]. In this way, the presence of empty Ln-4f bands is a possible reason for the low level of the conduction band edge. This empty 4f bands are not necessarily manifested as a drop in the band gap values, but these states can act as ‘stepping stones' for the electrons to jump to the conduction band [38]. Previous results on the photocatalytic activity of the layered tantalates RbLnTa2O7 (Ln = La, Pr, Nd, and Sm)[24],[39], with different cation arrangement between two contiguous perovskite slabs, account for the H2 evolution over the catalysts; 6, 4.2, and 47 for La, Pr, and Nd, respectively (all in units of mmol·h-1 for 1 g of catalyst). As a matter of comparison, when the NiOx loading was performed, the photocatalytic activity was notably improved, as reported by Shimizu et al. [9]. When NiOx was loaded onto the H2La2/3Ta2O7 and K2La2/3Ta2O7 surfaces, the hydrogen evolution increased by about ten times in the first compound (from 146 to 940 mmol·h-1). In the present work, the hydrogen evolution on the NiOx-LaN2 sample increased from 2.9 to 26.6 μmol h-1, and it was the highest increment observed in the photocatalytic activity.
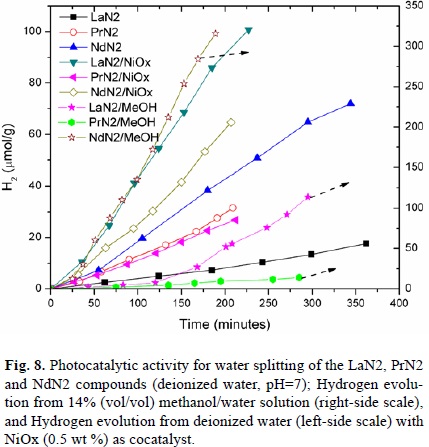
In order to explore the NiOx-cocatalyst effect on the H2 production of the LnN2 samples, NiOx-loaded LnN2 compounds were performed. The H2 evolution rates of the LaN2 and NdN2 samples were significantly increased by the addition of NiOx (0.5 wt %) as cocatalyst (Fig. 8, Table 5). In contrast to this behavior, the NiOx loading of the PrN2 sample resulted in a small decrease of the activity. However, the increase of NiOx-LaN2 activity was higher than the other compounds (from 2.92 µmol H2/g.h to 26.63 µmol H2/g.h). The higher activity of the NiOx-loaded compounds is due to the type of heterojunction formed at the semiconductor interface[40]. When NiOx and LnN2 are brought into contact, their Fermi levels align, due to the charge transfer phenomenon. Therefore, under illumination, the LnN2 compounds produce electron diffusion across the depletion region to the NiOx for H2 evolution. When the NiOx cocatalyst is pretreated under H2 reduction conditions, and then a subsequent O2 oxidation, a NiO/Ni double layer structure is produced, and the electron transference to the photocatalyst active sites is facilitated.
Conclusions
By the polymeric complex method, we successfully synthesized isostructural layered perovskite tantalates which correspond to the hydrated phases of K2Ln2/3Ta2O2 (Ln=La, Pr, Nd). These compounds are 1/3 Ln-deficient Ruddlesden-Popper systems of layered tantalates. Rietveld structural refinements and HRTEM images confirm the synthesis of Ln-deficient double-perovskite tantalates. The K2Ln2/3Ta2O7 (Ln=La, Pr, Nd) systems have a large ability to intercalate water molecules. This has been experimentally proved to be important in water splitting. According to the absorption-desorption isotherms, the higher surface area was obtained in the NdN2 compound. The DRS plot shows maxima absorption at 380 nm (Pr) and 585 nm (Nd), and this seems to depend on the nature of the lanthanide (La, Pr, Nd) in the crystal structure. The higher photocatalytic activity was observed in the NdN2 compound and it increased when NiOx cocatalyst was loaded on the surface of powders. Finally, the H2 evolution was higher when the reaction was carried out in an aqueous methanol mixture, due to the sacrificial reagent effect (except to PrN2). Layered lanthanide tantalates, particularly the Nd layered tantalate, are suitable compounds to reach high photocatalytic activity in water splitting. Through the experiments of this work, the role played by the Ln-deficiencies in the crystal structure is not clear and further experiments are needed in this direction.
Experimental
Preparation of samples
Samples with the general formula K2Ln2/3Ta2O7, with Ln = La, Pr, and Nd, were prepared by the Pechini method [41]–[43] in which nitrates of the respective lanthanides (La(NO3)3.6H2O, Nd(NO3)3.6H2O and Pr(NO3)3.6H2O (all of them Aldrich, 99.9%); tantalum chloride (TaCl5, Aldrich, 99.99%); and potassium carbonate (K2CO3, Aldrich, 99.995%) were dissolved in methanol in the cationic proportion 2:2/3:2. An excess, 100 % of K2CO3, was added to compensate for the volatility of the oxide form at high temperature. Then, the temperature was increased to 80°C and ethylene glycol was added to facilitate the solubility of salts. The solution was fully translucent and free of precipitates and suspended particles. At this step and with vigorous stirring, citric acid was added to enhance the solubility and form a condensation polymer until the solution became a viscous brown gel. These gels were slowly heated in a high-alumina crucible to 450°C for two hours, then black, highly porous, solids were formed. These solids were then finely ground for a subsequent calcination at 850°C for 48 hours. Subsequently, the compounds were suspended in water with stirring for approximately 30 minutes to dissolve the potassium excess and obtain the hydrated phases. The resulting HPs of the lanthanide tantalates were K2La2/3Ta2O7 (LaN2) (white powders); K2Nd2/3Ta2O2 (NdN2) (pale-purple powders) and K2Pr2/3Ta2O7 (PrN2) (pale-yellow powders). As previously reported by Crosnier-Lopez[27] for K2La2/3Ta2O7, the Ln-tantalates undergo rapid hydration when the samples are stored under room temperature conditions. Therefore, the crystal structure, X-ray diffraction (XRD), High Resolution Transmission Electron Microscopy (HRTEM)), specific surface area, thermal analysis, and photocatalytic activity characterization of the samples of this work, correspond to the hydrated form of the lanthanide tantalates, K2Ln2/3Ta2O7 (LnN2) (Ln=La, Pr, Nd). NiOx-Loaded catalysts were prepared by impregnation of the samples (2g) with an aqueous solution of Ni(NO3)2.6H2O (Aldrich, 99.99%). After stirring for 30 minutes, the water was evaporated in an oven. The powders were dried at 80°Cfor 4 h and then calcined at 350°C for 1 h, and then at 450°C for 2 h in air. Subsequently, the catalysts were reduced in a H2 atmosphere at 500 °C for 2 h, and oxidized in O2 atmosphere at 200°C for 1 h to produce NiO/Ni clusters (NiOx).
Characterization
XRD patterns of powders were gathered using a Siemens D-5000 diffractometer in a Bragg-Brentano geometry configuration (CuKα1 radiation, λ = 1.5406 Å), in air at room temperature (RT), with the operating conditions of 35kV and 35 mA. For the three hydrated compounds, the scanning angular range was 6°≤2θ≤90° with a step scan of 0.02°/10 seconds. Rietveld analyses were performed using the General Structure Analysis System[44] (GSAS Package) code with the graphical user interface EXPGUI[45]. After full hydration of the samples by continuous washing with deionized water, thermogravimetric analyses (TGA) were performed using a TGA Q50 (TA Instruments) with a heating rate of 10°C/min in airflow, from RT to 1000°C.
HRTEM analyses of hydrated samples were performed using a JEOL 2010-TEM/STEM microscope operated at 200 kV. The samples were softly ground to obtain fine powders and then dispersed in deionized water using an ultrasonic bath. A drop of this suspension was placed on a carbon-coated copper grid. Crystallographic information for the compounds was derived from the HRTEM micrographs and studied and analyzed using the commercial Digital Micrograph computing programme[46]. The electronic spectra (diffuse reflectance) were measured on a Cary 5E UV-Vis-NIR spectrophotometer in the 250-700 nm range. To determine the surface area, N2 adsorption-desorption isotherms were obtained using a Bel Japan Minisorp-II instrument. All the samples were previously degassed at 200°C under vacuum conditions for 4 hours. The photocatalytic activity was measured by hydrogen evolution with an inner irradiation cell made of quartz. The catalyst (1 g) was dispersed in aqueous methanol solution (14%) and deionized water by magnetic stirring and was irradiated by a high pressure Hg lamp (400W). Amounts of evolved gases were analyzed by gas chromatography (Varian Aerograph 1400, Ar carrier, stainless steel column 40/60 mesh) through a gas sampler (3 cm3) which was directly connected to the reaction system to avoid any contamination from air.
Acknowledgements
The authors thank M in C. Cecilia Salcedo-Luna (USAI-FQUNAM) for her help in the XRD experiments. This work was partially supported by the PAPIIT projects IN- 118710 and IN-214313. HV-S gratefully acknowledges the financial support from DGAPA (UNAM).
References
1. Ruddlesden, S. N.; Popper, P. Acta Crystallogr. 1957, 10, 538-539. [ Links ]
2. Ollivier, P. J.; Mallouk, T. E. Chem. Mater. 1998, 10, 2585-2587. [ Links ]
3. Schaak, R. E.; Mallouk, T. E. Chem. Mater. 2002, 14, 1455-1471. [ Links ]
4. Toda, K.; Watanabe, J.; Sato, M. Mater. Res. Bull. 1996, 31, 1427-1435. [ Links ]
5. Toda, K.; Teranishi, T.; Ye, Z. G.; Sato, M.; Hinatsu, Y. Mater. Res. Bull. 1999, 34, 971-982. [ Links ]
6. Schaak, R. E.; Mallouk, T. E. Chem. Mater. 2000, 12, 3427-3434. [ Links ]
7. Shimizu, K.; Itoh, S.; Hatamachi, T.; Kitayama, Y.; Kodama, T. J. Mater. Chem. 2006, 16, 773. [ Links ]
8. Tahara, S.; Ichikawa, T.; Kajiwara, G.; Sugahara, Y. Chem. Mater. 2007, 19, 2352–2358. [ Links ]
9. Shimizu, K. I.; Itoh, S.; Hatamachi, T.; Kodama, T.; Sato, M.; Toda, K. Chem. Mater. 2005, 17, 5161–5166. [ Links ]
10. Mitsuyama, T.; Tsutsumi, A.; Sato, S.; Ikeue, K.; Machida, M. J. Solid State Chem. 2008, 181, 1419–1424. [ Links ]
11. Ida, S.; Okamoto, Y.; Hagiwara, H.; Ishihara, T. Catalysts 2013, 3, 1–10. [ Links ]
12. Schottenfeld, J. A.; Kobayashi, Y.; Wang, J.; Macdonald, D. D.; Mallouk, T. E. Chem. Mater. 2008, 20, 213–219. [ Links ]
13. Josepha, E. A.; Wiley, J. B. Topochemical Manipulation of Layered Perovskites, University of New Orleans Theses and Dissertations. 2011, Vol. PhD. [ Links ]
14. Lehtimäki, M.; Yamauchi, H.; Karppinen, M. J. Solid State Chem. 2013, 204, 95–101. [ Links ]
15. Gopalakrishnan, J.; Bhat, V. Inorg. Chem. 1987, 26, 4299–4301. [ Links ]
16. Shimizu, K.; Tsuji, Y.; Kawakami, M.; Toda, K.; Kodama, T.; Sato, M.; Kitayama, Y. Chem. Lett. 2002, No. 11, 1158-1159. [ Links ]
17. Shimizu, K.; Tsuji, Y.; Hatamachi, T.; Toda, K.; Kodama, T.; Sato, M.; Kitayama, Y. Phys. Chem. Chem. Phys. 2004, 6, 1064. [ Links ]
18. Kudo, A.; Miseki, Y. Chem. Soc. Rev. 2009, 38, 253-278. [ Links ]
19. Kudo, A.; Kato, H. Chem. Phys. Lett. 2000, 331, 373-377. [ Links ]
20. Kudo, A. Pure Appl. Chem. 2007, 79, 1917-1927. [ Links ]
21. Kudo, A.; Kato, H.; Tsuji, I. Chem. Lett. 2004, 33, 1534-1539. [ Links ]
22. Kato, H.; Kudo, A. Catal. Letters. 1999, 58, 153–155. [ Links ]
23. Kato, H.; Asakura, K.; Kudo, A. J. Am. Chem. Soc. 2003, 125, 3082–3089. [ Links ]
24. Machida, M.; Yabunaka, J.; Kijima, T. Chem. Mater. 2000, 12, 812–817. [ Links ]
25. Kudo, A.; Okutomi, H.; Kato, H. Chem. Lett. 2000, 1212-1213. [ Links ]
26. Le Berre, F.; Crosnier-Lopez, M. P.; Fourquet, J. L. Mater. Res. Bull. 2006, 41, 825–833. [ Links ]
27. Crosnier-Lopez, M. P.; Le Berre, F.; Fourquet, J. L. Z. Anorg. Allg. Chem 2002, 628, 2049–2056. [ Links ]
28. Le Berre, F.; Crosnier-Lopez, M. P.; Laligant, Y.; Fourquet, J. L. J. Mater. Chem. 2002, 12, 258–263. [ Links ]
29. Machida, M.; Miyazaki, K.; Matsushima, S.; Arai, M. J. Mater. Chem. 2003, 13, 1433. [ Links ]
30. Crosnier-Lopez, M. P.; Le Berre, F.; Fourquet, J. L. J. Mater. Chem. 2001, 11, 1146–1151. [ Links ]
31. Richard, M.; Brohan, L.; Tournoux, M. J. Solid State Chem. 1994, 112, 345–354. [ Links ]
32. Shannon, R. D. Acta Crystallogr. Sect. A. 1976, 32, 751-767. [ Links ]
33. Sing, K. S. W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure and Applied Chemistry, 1985, 57, 603–619. [ Links ]
34. Thommes, M. Chemie-Ingenieur-Technik. 2010, 82, 1059-1073. [ Links ]
35. Li, D.; Zheng, J.; Li, Z.; Fan, X.; Liu, L.; Zou, Z. Int. J. Photoenergy. 2007, 2007, 1-7. [ Links ]
36. Luan, J.; Cai, H.; Zheng, S.; Hao, X.; Luan, G.; Wu, X.; Zou, Z. Mater. Chem. Phys. 2007, 104, 119-124. [ Links ]
37. Machida, M.; Yabunaka, J.; Kijima, T.; Matsushima, S.; Arai, M. Int. J. Inorg. Mater. 2001, 3, 545-550. [ Links ]
38. Ramírez-De-Arellano, J. M.; Ruiz-Chavarría, S.; Valencia-Sánchez, H.; Tavizon, G.; De La Mora, P. Comput. Mater. Sci. 2014, 93, 160-163. [ Links ]
39. Machida, M.; Yabunaka, J.; Kijima, T. Chem. Commun. 1999, 1939–1940. [ Links ]
40. Hu, C. C.; Teng, H. J. Catal. 2010, 272, 1–8. [ Links ]
41. Yoshino, M.; Kakihana, M.; Cho, W.; Kato, H.; Kudo, A. Chem. Mater. 2002, 14, 3369–3376. [ Links ]
42. Takahashi, H.; Kakihana, M.; Yamashita, Y.; Yoshida, K.; Ikeda, S.; Hara, M.; Domen, K. J. Alloys Compd. 1999, 285, 77–81. [ Links ]
43. Ikeda, S.; Hara, M.; Kondo, J. N.; Domen, K.; Takahashi, H.; Okubo, T.; Kakihana, M. Chem. Mater. 1998, 10, 72–77. [ Links ]
44. Larson Von Dreele, R.B, a C. General Structure Analysis System (GSAS), 1994, Los Alamos National Laboratory. [ Links ]
45. Toby, B. H. J. Appl. Crystallogr. 2001, 34, 210–213. [ Links ]
46. Team, G. S. DigitalMicrograph 3.7.0 for GMS 1.2, 1999. [ Links ]














