Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.59 no.1 Ciudad de México ene./mar. 2015
Article
Optimization of Extraction Conditions for Flavonoids of Physalis alkekengi var. franchetii Stems by Response Surface Methodology and Inhibition of Acetylcholinesterase Activity
Xue-gui Liu,1 Fu-yu Jiang,1 Pin-yi Gao,1,* Mei Jin,2 Di Yang,1 Zhong-feng Nian,2 and Zhen-xue Zhang1
1 College of Pharmaceutical and Biological Engineering, Shenyang University of Chemical Technology, Shenyang, Liaoning 110142, P.R. China. liuxuegui72@163.com.
2 College of Chemical Engineering, Shenyang University of Chemical Technology, Shenyang, Liaoning 110142, P.R.China.
Received October 3rd, 2014
Accepted December 11th, 2014
Abstract
The microwave-assisted extraction conditions of flavonoids in Physalis alkekengi. var. franchetii stems were optimized using a L33 Box-Behnken Design. The optimized extraction conditions were determined as follows: 60% for the ethanol concentration, 12.4 for the liquid-to-solid ratio, and 531.4 W for the microwave power, respectively. The yield of the extract obtained under the optimized conditions was 3.85 mg g-1 which was close to the predicted value. In addition, the extract exhibited potent acetylcholinesterase inhibitory activity, with the IC50 value of 11.61 μg mL-1 and a maximal inhibition ratio 89.81%. Both the yield and activity were better than reflux extraction.
Key words: Physalis alkekengi. var. franchetii Stems; Flavonoids; Microwave-assisted Extraction; Response Surface Methodology; Acetylcholinesterase inhibitors.
Resumen
En esta investigación se optimizaron las condiciones de extracción de flavonoides a partir de Physalis alkekengi var. Franchetii, asistida por microondas, usando un diseño L33 Box-Behenke. Las condiciones determinadas fueron etanol 60%, la relación líquido:sólido fue de 12.4 y se usaron 531.4 W para el microondas. El rendimiento obtenido fue de 3.85 mg g-1, el cual es cercano al valor predicho. El extracto obtenido inhibe de forma importante la actividad de la acetilcoliestearasa, con una IC50 de 11.61 µg mL-1 y una inhibición máxima de 89.91%. Estos resultados son mejores que los obtenidos con el compuesto obtenido mediante extracción por reflujo.
Palabras clave: Acetilcolín estearasa, extración por microondas, flavonoides, inhibición, Physalis alkekengi.
Introduction
Physalis alkekengi. var. franchetii (Solanaceae) is a traditional Chinese herbal medicine which has been reported to exhibit a variety of pharmaceutical effects, such as antitumor [1] and antimalarial [2], as well as antimycobacterial, anti-inflammatory actions [3]. More recently, a variety of bioactive compounds were reported from Physalis alkekengi. var. franchetii, such as, steroids, sterols [4], flavonoids, alkaloids, and inorganic elements [5]. As one of main constituents of Physalis alkekengi. var. franchetii[6], flavonoids exhibit a variety of physiological effects including anti-influenz [7], immunomodulatory, anti-inflammatory [8], anticancer[9], antioxidant and free radical scavenging properties[10] and acetylcholinesterase inhibitory activity[11]. Flavonoids are existing widely in plants and more than 8000 different flavonoids have been identified [12]. Many of them have been applied in the field of nutraceuticals and pharmaceuticals. Therefore, the flavonoids in Physalis alkekengi. var. franchetii have the potential for commercial application.
Acetylcholinesterase is the enzyme that hydrolyzes acetylcholine in the cholinergic nervous system. However, it can cause Alzheimer's disease (AD) if present in high concentrations [13]. The most common symptoms include aphasia and memory impairment [14]. Acetylcholesterase inhibitors are widely used for the treatment of AD and these can be divided into chemically synthesized and natural products. Both galanthamine and huperzine A, natural products that exhibit effective acetylcholinesterase inhibitory activity[15]. Bioactive screening of acetylcholinesterase inhibitors by Ellman's was high-performance method in phytochemistry area, and a number of researchers have become interested in acetylcholinesterase inhibitors and bioassay-guided fractionation for natural products.
The extraction method used is an important factor in any analysis of the chemical constituents. Microwave-assisted extraction is widely used for extracting biologically active compounds from different natural products. Ionic conduction and dipole rotation are the principles of heating by microwave energy [16]. On the basis of the principles and study reports, microwave-assisted extraction is able to significantly reduce the extraction time, the amount of organic solvents required and increase the yields compared with conventional extraction techniques [17]. In addition, response surface methodology (RSM) is one of the most widely applied statistical methods. It has a high degree of accuracy for determining the relationship between various parameters and responses and examining the interaction between variables. The Box-Bhenken design (BBD) was used for designing the experiments to evaluate the nonlinear relationship between response values and factors. It has the advantage of being able to reduce the combinations compared with the other designs [18].
The inhibition of acetylcholinesterase activity and research potential for flavonoids of Physalis alkekengi. var. franchetii stems prompted us to examine studies to the microwave-assisted extraction conditions by Response Surface Methodology based on single factorial experiments to select five conditions for optimizing the procedure (ethanol concentration, extraction time, number of extractions, liquid-to-solid ratio, and microwave power). In this study, the objective was to investigate the extraction conditions for flavonoids and inhibition of acetylcholinesterase activity of Physalis alkekengi. var. franchetii stems. Finally, the optimized extraction conditions were considered for application on a laboratory or industrial scale.
Results and Discussion
Effects of the ethanol concentration
The ethanol concentration ranged from 50% to 70%, and the other extraction conditions were fixed as follows: extraction time 12 min, number of extractions two, liquid-to-solid ratio 12, and microwave power 440 W. The yields of flavonoids gradually increased and when the ethanol concentration reached 70%, the flavonoid yield reached a maximum. Also, as the ethanol concentration increased the extraction yields fell markedly (Fig. 1A). Hence, the ethanol concentration has an important influence on the yield.
Effects of the extraction time
This study was carried out to examine the effect of the extraction time on the extraction efficiency (Fig. 1B). A range of extraction times (4 min, 6 min, 8 min, 10 min, 12 min) were selected for the experiments, with the other extraction conditions fixed as follows: ethanol concentration 70%, number of extractions two, liquid-to-solid ratio 12 and microwave power 440 W. From 4 min to 10 min, the yields increased slightly. However, the yield fell when the extraction time reached 12 min, but the change was not significant. Solvent evaporation was the reason for this. Therefore, the extraction time was set at 10 min in further experiments.
Effects of the number of extractions
To investigate the effect of the number of extractions on the yield of flavonoids, the number of extractions was carried out from one to five times (Fig. 1C), while the other extraction conditions was fixed as follows: ethanol concentration 70%, extraction time 12 min, liquid-to-solid ratio 12 and microwave power 440 W. As the number of extractions increased the yield of flavonoids increased. However, there was no significant increase between two from five extractions. Furthermore, the experimental cost of five extractios was substantial for industrial scale compared with two extractions. Meanwhile, the average time needed for each extraction was approximately 25 min and so the five extractions took 75 min longer than two extractions. Therefore, to save manpower, reduce the cost, and save time, two extractions were found to be most appropriate.
Effects of the liquid-to-solid ratio
The effect of liquid-to-solid ratio (Fig. 1D) was found to have a highly significant effect on the yield of the flavonoids in Physalis alkekengi.var. franchetii stems, the other extraction conditions was fixed as follows: ethanol concentration 70%, extraction time 12 min, number of extractions two and microwave power 440 W. Increasing the liquid-to-solid ratio from 8 to 12 led to a marked increase in the extraction yield. If the liquid-to-solid ratio exceeded 12, the extraction yields were markedly reduced. The possible reason for this is that too high a liquid-to-solid ratio leads to microwave energy being dissipated by the shaking mixture and excessive swelling of plant materials[16, 19].
Effects of the microwave power
To examine the effect of microwave power on the extraction efficiency (Fig. 1E), the microwave power was set at 80 W, 240 W, 440 W, 640 W and 800 W, while the other extraction conditions were fixed as follows: ethanol concentration 70%, extraction time 12min, number of extractions two, liquid-to-solid ratio 12. It was observed that the yields markedly increased over the range from 80 W to 440 W but then the yield fell markedly when the microwave power exceeded 440 W. If the microwave power was too high it decomposed the thermolabile flavonoids, thereby leading to a reduced yield [20]. Therefore, the microwave power is a main factor in the procedure.
Optimization of microwave-assisted extraction conditions of flavonoids by RSM
According to the modeling and statistical analyses (Tables 1 and 2) using Design Expert 8, data were analyzed to fit the following polynomial equation to Y (the yields of flavonoids in Physalis alkekengi. var. franchetii stems).
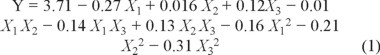
where X1 is the ethanol concentration (%), X2 is the liquid-to-solid ratio (mL g-1), and X3 is the microwave power (W).
The p value (0.02) of the model was lower than 0.05 (Table 3), indicating that the model fitness was significant. Also, the lack of fit was not significant, the coefficient of determination (R2) was 0.8708. Thus, the experimental model was adequate. The p value (p < 0.01) of the ethanol concentration indicated that the ethanol concentration have a highly significant influence on flavonoid yields [21]. However, the p value of liquid-to-solid ratio (p > 0.05) and microwave power (p > 0.05) have not significant influence on yields.
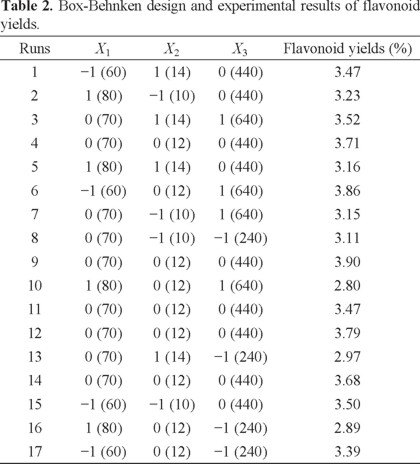
The two-dimensional contour plots and three-dimensional response surface was illustrated for each of the factors and interactions. The ethanol concentration and liquid-to-solid ratio had an effect on yield of flavonoids (Figs. 2A and 3A). If the value of liquid-to-solid ratio was in the middle and the ethanol concentration was in the lower region, the yield exhibited a significant increase. However, as the ethanol concentration increased, the yield began to decrease [22]. The interactions between the ethanol concentration and the liquid-to-solid ratio was not significant [23]. The interactions of the ethanol concentration and microwave power shown clearly affected the yield (Figs. 2B and 3B). With an increase in ethanol concentration increased if the microwave power was low. However, the yield was reduced at a higher microwave power but this was not significant [24]. On increasing the microwave power, an increase in the liquid-to-solid ratio resulted in the maximum yield. However, the trend was reversed with a further increase in the liquid-to-solid ratio and microwave power (Figs. 2C and 3C).
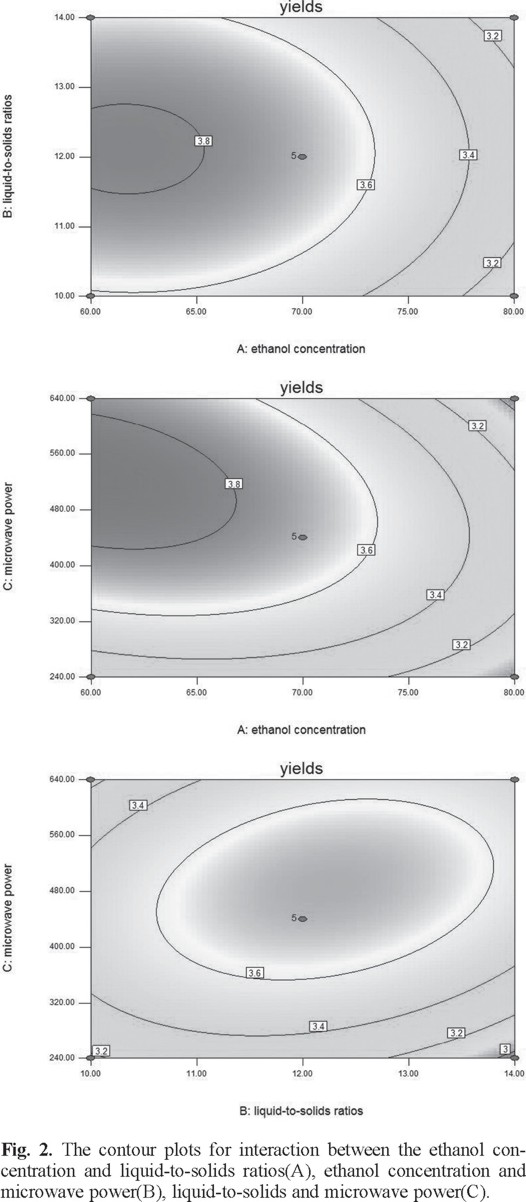
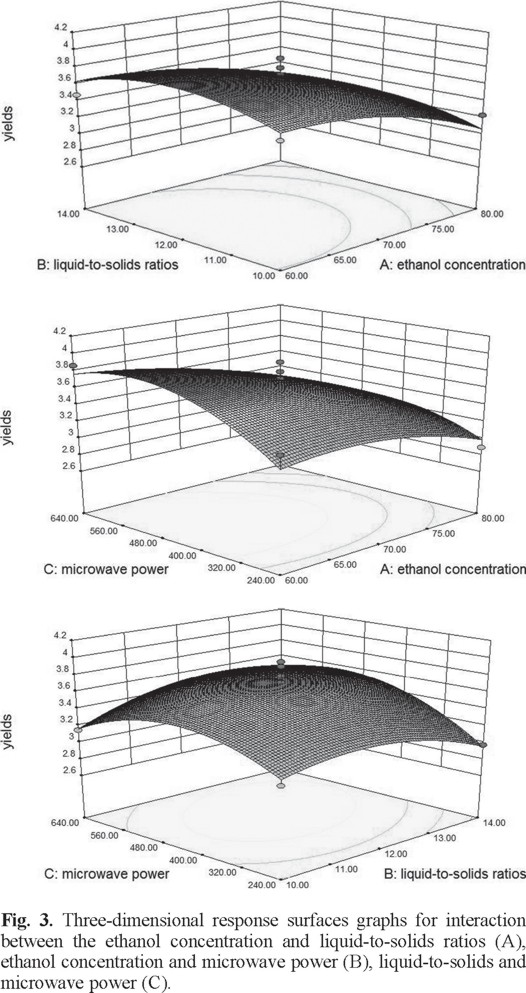
Ultimately, the optimum values for each of the factors were 60% for the ethanol concentration, 12.4 for the liquid-to-solid ratio, and 531.4 W for the microwave power. The experimental value to predict of the extraction yield of flavonoids under optimized conditions was 3.87 mg g-1.
Experiment validation
In order to validate the suitability of the model to predict the extraction yield, the experiment was carried out at a 60% ethanol concentration, 12.4 for liquid-to-solid ratio, and 531.4 W for microwave power. Also three replicates were carried out under the conditions chosen. The results showed that the average maximum yield was 3.85 mg g-1 which was close to the predicted value and its RSD was 1.2% (lower than 5%). Therefore, the microwave-assisted extraction conditions for flavonoids of Physalis alkekengi. var. franchetii stems optimized by BBD are both accurate and reliable [25].
Inhibition of acetylcholinesterase activity
According to the results calculated by SPSS 18.0 for the inhibition of acetylcholinesterase activity assay, the IC50 value of the extracts under the optimized conditions is 11.61 μg mL-1 and the maximal inhibition ratio was 89.81% (Table 4 and Fig. 4) [26]. Hence, the extracts under optimized conditions were good effective in inhibiting acetylcholinesterase. The results obtained suggest that flavonoids play important role in acetylcholinesterase inhibitors.[27].
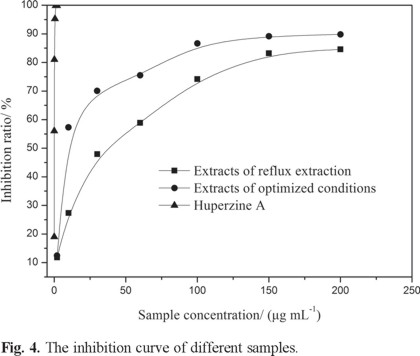
Comparison of extract under optimized microwave-assised extraction conditions with reflux extraction
The reflux extraction fixed as follows: ethanol concentration 60%, extraction time 1h, number of extractions two, liquid-to-solid ratio 12 and temperature of reflux 70 °C. The average yields was 3.24 mg g-1, with an IC50 value of 29.97 μg mL-1 and a maximum inhibition ratio of 84.58% (Table 4). The results showed that the yield and activity of microwave-assisted extraction under optimized conditions were increases compare with reflux extraction (Table 5 and Fig. 4). So the yield and activity under optimized microwave-assisted extraction conditions are better than reflux extraction.
Conclusion
Optimization of the microwave-assisted extraction conditions for flavonoids of Physalis alkekengi. var. franchetii stems by using the BBD of the three-factor three-level system on the basis of a single factor is both appropriate and accurate. The result of experiment validation showed that the data was close to the predicted value. Meanwhile, the extract under optimized microwave-assisted extraction conditions was good effect in inhibition of acetylcholinesterase activity and flavonoids play an important role in acetylcholinesterase inhibitors. Furthermore, microwave-assisted extraction under optimized conditions compare with reflux extraction in terms of yield and activity are better. In conclusion, the results can provide a theoretical basis for bioassay-guided fractionation of acetylcholinesterase inhibitory activity for the flavonoids of Physalis alkekengi. var. franchetii stems.
Experimental
Extraction of flavonoids
Dried Physalis alkekengi. var. franchetii stems were finely crushed and 2g samples of the powder were used. Factorial experiments were used to examine the experimental conditions [28], and involving the microwave-assisted extraction applied to different parameters including ethanol concentration, extraction time, number of extractions, liquid-to-solid ratio, as well as the microwave irradiation power. The extracts were filtered under vacuum, enriched by rotary evaporation and collected in a volumetric flask [29]. Furthermore, an experiment of comparison by reflux extraction was conducted.
Standard curve of flavonoids
The yields of flavonoids in the extracts of Physalis alkekengi. var. franchetii stems were measured with a chromogenic system of NaNO2-Al(NO3)3-NaOH. In these studies, Rutin was used as a standard and stock solutions of rutin (0.20 mg mL-1) of were prepared in methanol [30]. Then, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0 mL samples of the rutin standard stock solution of and water respectively was placed in 25 mL volumetric flasks. Then, 1mL 5% sodium nitrite solution was added to each flask followed by shaking, and the flasks were then allowed to stand for 6 min. This was followed by the addition of 1 mL 10% aluminum chloride with shaking. After standing for another 6 min, 1mL 5% sodium hydroxide was added. A series of standard solutions of the flavonoids at a concentration of 0.008, 0.016, 0.024, 0.032, 0.040 and 0.048 mg mL-1 were prepared by appropriate dilution of the stock solutions with methanol. The absorbance of each solution was measured UV-VIS-NIR spectroscopy at 510 nm and drawn a curve of rutin was obtained. The extraction yield of flavonoids in the extracts of Physalis alkekengi. var. franchetii stems was determined using this standard curve. The linear regression equation of the standard curve was A510 = 9.9571 C- 0.0188, R2 = 0.9997, where A510 is the absorbance at 510 nm and the C is the concentration of rutin (mg mL-1), with the concentration of flavonoids ranging from 0.008 to 0.048 mg mL-1. The yields of flavonoids in the Physalis alkekengi. var. franchetii stems were obtained as follows:
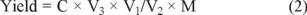
where C is the flavonoid concentration of the diluted samples (mg mL-1), V1 is the constant volume of the solvent of the extracts to be measured, V2 is the volume of the solution that was diluted (mL), and V3 is the constant volume of solution to be tested (mL). M is the weight of the powder sample of Physalis alkekengi. var. franchetii stems.
Experimental design
Optimizing microwave-assisted extraction conditions was carried out by three-factor three-level BBD based on the single factor experimental results to examine the effect of different parameters, namely, the ethanol concentration, the extraction time, the number of extractions, the liquid-to-solid ratio, as well as microwave irradiation power on the extraction yields of flavonoids in the Physalis alkekengi. var. franchetii stems [31].
The independent variables were coded at three levels (-1, 0, and 1) and the each factor and level was selected on the basis of preliminary single experiments for a suitable range [32]. The complete experimental design consisted of 17 experimental points. The independent variable xi was coded as Xi according to equation 1.
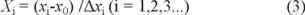
where Xi is the coded value of an independent variable, xi is the actual value of an independent variable, x0 is the actual value of an independent variable at the center point, and Δxi is the value of the step change.
This design was conducted using a second-order polynomial model involving the response surface regression procedure of a statistical analysis system based on Box-Bhenken model, The form of polynomial equation 2 was as follows.
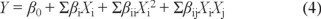
where Y is the predicted response, βi, βii, Σβii and Σβij are the regression coefficients for the intercept, linearity, square and interaction, respectively, while Xi and Xj are independent variables [33].
Determination of the inhibition of acetylcholinesterase activity
The substrate acetylthiocholine was hydrolyzed by acetylcholinesterase to produce thiocholine , then the thiocholine interacted with DTNB to give a yellow color. Using an Ellman assay, the half maximal inhibitory concentration (IC50) was calculated for extracts with a range of concentrations (2, 10, 30, 60, 100, 150, 200 μg mL-1) [34] on the optimized conditions and Huperzine A was used as a standard.
The each of the wells of 96-microtiter well plates was added 140 μL phosphate buffer (0.1 M pH 7.4), 20 μL of different concentrations of sample, 15 μL acetylcholinesterase (0.4 U mL-1), followed by incubating for 20 min at 4 °C. Then, 10 μL ATCI and 20 μL DTNB were added, followed by incubating for 30 min at 37 °C. The absorbance of each sample (As) was measured at 405 nm using a microplate reader [35]. The blank group contained all components except sample (A0), the background group (Ab) contain all components except acetylcholinesterase, complete inhibition group was 100 μg mL-1 Huperzine-A (Ac) was used as the substitute sample. The inhibition ratio was calculated using the equation.
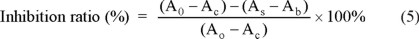
Acknowledgments
The authors are grateful to the Projects is sponsored by Liaoning BaiQianWan Talents Program (Grant No. 2013921048) and Science and Technology Department of Liaoning Province (Grant No. 2013010397-401).
References
1. Zhang, C. H.; Wang, Z. T.; Yang, Y. P.; Sun, S. Q. Chinese Chem. Lett. 2009, 11, 1327-1330. [ Links ]
2. Matheus, S. S.; Maria, N. de Menezes.; Antoniana, U.; Ivone, M. R.; Therezinha, C. B. T.; Ricardo, Ribeiro dos Santos.; Walter F, de Azevedo.; Milena, B. P. S. J. Agr. Food Chem. 2011, 74, 2269-2272. [ Links ]
3. Ji, L.; Yuan, Y. L.; Luo, L. P.; Chen, Z.; Ma, X. Q.; Ma, Z. J.; Cheng, L. Steroids. 2012, 5, 441-447. [ Links ]
4. Zheng, Y. L.; Liu, X. S.; Luan, L. J.; Wang, L. H.; Wu, Y. J. K. J. Chem. Eng. Data. 2010, 55, 3690-3692. [ Links ]
5. Chen, L. X.; Xia, G. Y.; Liu, Q. Y.; Xie, Y. Y.; Qiu, F. Syst. Ecol. 2014, 54, 31-34. [ Links ]
6. Qiu, L.; Zhao, F.; Jiang, Z. H.; Chen, L. X.; Zhao, Q.; Liu, H. X.; Yao, X. S.; Qiu, F. J. Nat. Prod. 2008, 71, 642-646. [ Links ]
7. Hisahiro, K.; Masatsugu, O.; Hiroki, Y.; Wataru, W.; Shigetoshi, T.; Park, Y. K.; Matsuno, K. J.; Yasukawa. K.; Masahiko, K. J. Funct. Foods. 2014, 5, 214-220. [ Links ]
8. Alcaraz, M. J.; Hoult, J. R. Biochem. Pharmacol. 1985, 34, 2477-2480. [ Links ]
9. Divyashree, R.; Amit, K. R.; Francesca, G.; Helen. M. I. O. Int. J. Biochem. Cell. B. 2013, 12, 2821-2829. [ Links ]
10. Kelly, E. H.; Anthony, R. T.; Dennis, J. B. J. Nutr. Biochem. 2002, 10, 572-584. [ Links ]
11. Jung, M.; Park, M. S. Molecules. 2007, 12, 2030-2132. [ Links ]
12. Moon, Y. J.; Wang, X. D.; Morris, M. E. Toxicol. in Vitro. 2006, 20, 187-210. [ Links ]
13. Pohanka, M.; Jun, D.; Kuca, K. J. Enzym. Inhib. Med.Ch. 2009, 24, 3680-3681. [ Links ]
14. Sutovsky, S.; Blaho, A.; Kollar, B.; Siarnik, P.; Csefalvay, Z.; Dragasek, J.; Turcani, P. Bratisl. Med. J. 2014, 115, 116-117. [ Links ]
15. Ibrahim, F.; Andre, C.; Thomassin, M.; Guillaume, Y. C. J. Pharmaceut. Biomed. 2008, 48, 1345-1346. [ Links ]
16. Zhang, H. F.; Yang, X. H.; Wang Y. Trends. Food Sci. Tech. 2011, 22, 672-682. [ Links ]
17. Chi, Y. S.; Zhang, Z. D.; Li, C. P.; Liu, Q. S.; Yan, P. F.; Urs, W. B. Green Chem. 2011, 13, 666. [ Links ]
18. Yadollah, B.; Heshmat, E.; Farshad, F-Far. Org. Process Res. Dev. 2012, 16, 1733-1738. [ Links ]
19. Xu, W.; Chu, K. D.; Li, H.; Zhang, Y. Q.; Zheng, H. Y.; Chen, R. L.; Chen, L. D. Molecules. 2012, 17, 14331. [ Links ]
20. Kadam, S. U.; Tiwari, B. K.; O'Donnell, C. P. J. Agr. Food Chem. 2013, 61, 4667-4675. [ Links ]
21. Winny, R.; Valérie, O. Food Bioprocess Tech. 2012, 5, 409-424. [ Links ]
22. Wang, L. Z.; Yang, B.; Du, X. Q.; Yi, C. Food Chem. 2008, 108, 737-741. [ Links ]
23. Liu, Z. G.; Mei, L. J.; Wang, Q. L.; Shao, Y.; Tao. Y. D. LWT-Food Sci. Technol. 2014, 56, 171-172. [ Links ]
24. Zhang, H. W.; Bai, X. L. J. Food Sci. Technol. 2014, 51, 371-376. [ Links ]
25. Nino, L. R.; Grosseli, G. M.; Mozeto, A. A.; Fadini, P. S. Anal. Methods. 2014, 6, 3247-3253. [ Links ]
26. Fallarero, A.; Oinonen, P.; Gupta, S.; Blom, P.; Galkin, A.; Mohan, C. G.; Vuorela, P. M. Pharmacol. Res. 2008, 58, 219-221. [ Links ]
27. Saviana, D. G.; Aline, B.; Aurelie, U.; Andrew, M.; Kurt, H.; Pierre, A. P.; Marianne, R. Eur. J. Pharm. Sci. 2008, 33, 109-111. [ Links ]
28. Zhang, D. Y.; Yao, X. H.; Duan, M. H.; Luo, M.; Wang, W.; Fu, Y. J.; Zu, Y. G.; Efferth, T. Andr. Analyst. 2013, 138, 4631-4641. [ Links ]
29. Li, Y. L., Fang, Z. X.; You, J. J. Agr. Food Chem. 2013, 61, 1467-1469. [ Links ]
30. Kim, M. B.; Park, J. E.; Woo, S. W.; Lim, S. B.; Hwang, J. K. Food Sci. Biotechnol. 2014, 23, 736-738. [ Links ]
31. Bimakr, M.; Abdul, R. R.; Ganjloo, A.; Taip, F. S.; Salleh, L. M.; Sarker, M. Z. I. Food Bioprocess Tech. 2012, 5, 916-919. [ Links ]
32. Noe, S-Gonzalez.; Monica, R. J-Fonseca.; Eduardo, S. M-Martinez.; L Gerardo, Z. J. Agr. Food Chem. 2013, 61, 11998-11999. [ Links ]
33. Mariutti, L. R. B.; Nogueira, G. C.; Bragagnolo, N. J. Agr. Food Chem. 2008, 56, 2913-2918. [ Links ]
34. Holth, T. F.; Tollefsen, K. E. Aquat. Toxicol. 2012, 112-113, 96-97. [ Links ]
35. Guo, A. J. Y.; Xie, H. Q.; Choi, R. C. Y.; Zheng, K. Y. Z.; Bi, C. W. C.; Xu, S. L.; Dong, T. T. X.; Tsim, K. W. K. Chem-Biol. Interact. 2010, 187, 246-248. [ Links ]














