Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.59 no.1 Ciudad de México ene./mar. 2015
Article
Stereochemical Investigation of a Novel Biological Active Substance from the Secondary Metabolites of Marine Fungus Penicillium chrysogenum SYP-F-2720
Linwei Li,1 Yalu Zhang,1 Zhen Li,1 Zhiguo Yu,2 and Tiemin Sun1,*
1 Key Laboratory of Structure-Based Drug Design and Discovery, Shenyang Pharmaceutical University, Ministry of Education. Shenyang 110016, PR China. suntiemin@126.com
2 Pharmacy Department, Shenyang Pharmaceutical University. Shenyang 110016, PR China.
Received November 11th, 2014
Accepted December 4th, 2014
Abstract
The chemical structure and absolute configuration of a novel benzoic acid (1) which is the secondary metabolites from the marine fungus Penicillium chrysogenum SYP-F-2720, has been determined by experimental spectroscopic data and quantum chemical calculations of its electronic circular dichroism (ECD). The configurational assignments were further confirmed by the highly consistent spectra between natural compound and synthetic compound which from raw material with a definite configuration. Furthermore, The target compound exhibited more significant anti-inflammatory and analgesic activities than aspirin when administered at 100 mg/kg, however, it behaved no ulcerogenic effect.
Key words: (S)-2-(2-hydroxypropanamido) Benzoic Acid, Structural Elucidation, DFT, ECD, Anti-inflammatory.
Resumen
Se determinó la estructura química y la configuración absoluta de un ácido benzoico novedoso (1), presente en los metabolitos secundarios del hongo marino Penicillium chrysogenum SYP-F-2720. La determinación se llevó a cabo mediante datos espectroscópicos experimentales y cálculos químicos cuánticos de su dicroísmo circular electrónico (DCE). La asignación de la configuración se confirmó mediante la comparación de los espectros del compuesto natural y el correspondiente compuesto sintético. Además, el compuesto aislado exhibe actividades anti-inflamatorias y analgésicas más significativas que la aspirina cuando se administró a una dosis de 100 mg / kg, y no mostró ningún efecto ulcerogénico.
Palabras clave: (S)-2-(2-hidroxipropanamido) Benzoico, elucidación estructural, DFT, ECD, antiinflamatorio.
Introduction
The secondary metabolites of marine microorganisms is fairly recognized as a potential source of original structure and biological activity substance due to its strain-specific [1-5]. Marine fungus is a class of marine microorganisms, and its secondary metabolites demonstrate biodiversity [6]. As the most primitive multicultural marine fungus organisms, sponge is an important source of natural active ingredients [7, 8]. To data, numerous novel biological active substance with antitumor, antiviral, antibacterial, antiinflammatory, and enzyme inhibitory activities have been isolated from sponge-derived marine fungus, such as aspergione A-F from Aspergillus versicolor, evariquinone and isoemericellin from Emericella variecolor, isocyclocitrinol A from Penicillium citrinum, sorbicillactones A from Penicillium chrysogenum [7-12]. SYP-F-2720 is a sponge-derived marine fungus belongs to P. chrysogenum strain, which is no relevant research on it so far. In the course of a program aiming at the isolation of secondary metabolites from the fermentations of P. chrysogenum, a novel benzoic acid (compound 1) was recently isolated from the secondary metabolites of P. chrysogenum SYP-F-2720 together with three known compounds, cycol-(Pro-Leu), cyclo-(4-hydroxyl-Pro-Phe), (22E,24R)-Ergosta 5,7,22-triene-3β-ol [13-15]. Yet its absolute configuration remained unknown. Herein, we describe the absolute stereochemistry of compound 1 by means of experimental spectroscopic data and quantum chemical calculations. Configuration assignment of the target compound was further verified by synthetic compound which from raw material with a definite configuration. Furthermore, the anti-inflammatory and analgesic activities of 1 were evaluated.
Results and Discussion
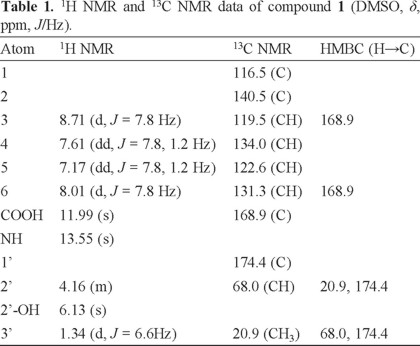
The chemical structure of compound 1 (Fig. 1) was elucidated by a more detailed 1H and 13C NMR spectroscopic analysis via 1H1H COSY, HMBC, HSQC and DEPT experimental finding (Table 1). 1H NMR spectrum of compound 1 implied that four aromatic proton signals at δH 7.17 (td, J = 7.8, 1.2 Hz), 7.61 (td, J = 7.8, 1.2 Hz) and δ 8.01 (dd, J = 7.8, 1.2 Hz), 8.71 (dd, J = 7.8, 1.2 Hz) which were assigned to a an ortho-substituted benzene ring, besides, two hydroxyl groups at δH 6.13 (2'-OH, s) and δH 11.99 (COOH, s), one methine (δH 4.16, m), one methyl group (δH 1.34, d) and amino proton at δH 13.55 were also detected. The fact that the carboxylic acid group connected to the phenyl group was confirmed by the resonance at δc 168.9 ppm in the 13C NMR, the absorption bands at 3400.4 and 1660.6 cm-1 in IR spectrum, which suggested a conjugated nature, and also supported by the HMBC correlation between H-3, H-6 and δc 168.9. The 1H-1H COSY spectrum of the title compound demonstrated the presence of two spin systems (H-2'─H3-3' and H-2'─H-2'-OH) (Fig. 2). Associated with the HMBC correlations between H-3' and C-2', 1', H-2' and C-3', 1' illustrated the presence of CH3-CH-C = O. The structure assigned to 1 was also confirmed by its HR-EI-MS (m/z [M + Na] 232.0580, calcd for C10H11O4N 209.0581).
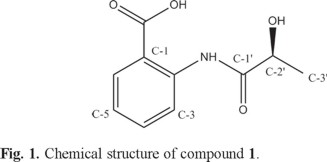
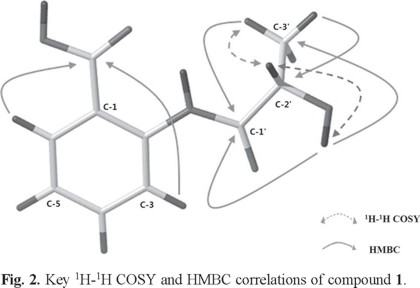
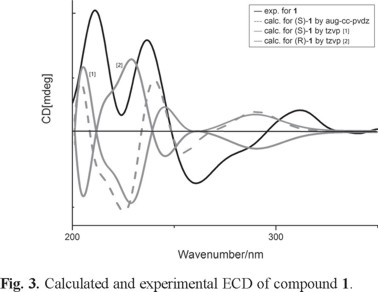
The stereochemistry assignment of compound 1 was determined by electronic circular dichroism (ECD) spectra provided by measured and quantum chemical calculations which carried out by TD-DFT/CAM-B3LYP/TZVP [16, 17]. The spectrum simulated for (S)-1, reproduced the sign and intensity of measured positive CE at 237 and negative CE at 261 nm, is in excellent agreement with the experimental pattern. On the contrary, the calculated ECD spectrum for (R)-1 was almost opposite to the experimental curve of (S)-1. Thus, the absolute configuration of 1 was assigned to S.
In order to get reliable results, TD-DFT/CAM-B3LYP/aug-cc-pVDZ ECD calculations were also performed for the conformers of (S)-1 [18]. The Boltzmann-weighted ECD of (S)-1 by different basis sets did not lead to notable changes in the ECD curve profile (Fig. 3).
The origin of the CEs in the experimental ECD of 1 could be explained by molecular orbital (MO) analysis at the same level of ECD calculation (Fig. 4). As inferred from the MO analysis, the significant positive CE at 211 nm was contributed by the electronic transition (ET) from MO 54 to MO 57 involving the π→π* transition of phenyl ring and carbonyl group n→π* transition. The positive CE at 237 nm is mainly dominated by transitions from MO 54/MO 56 (50%) and MO 55/MO 57 (42%), which could be ascribed to the combination of a benzene ring π→π* transition and the n→π* transition of amide and hydroxy groups. Moreover, the strong negative CE at 261 nm in the measured spectrum also derived from the same ET which causing the CE at 237 nm. According to the above data, it was suggested that the CEs at 211, 237, 261 and 312 nm in the ECD curve were determined by the configurational assignment of C-2', which was in accordance with the theoretical ECD spectra for the two stereisomrs of (R and S).
In our research, compound 1 has been synthesized from commercially available chiral precursors. Synthetic compound presented remarkable similar spectroscopic parameters to compound 1, particularly ECD spectral features (Fig. 3). The synthetic procedure of compound 1 is shown in Scheme 1.

On the basis of the above evidence, the complete structure of compound 1 was elucidated as (S)-2-(2-hydroxypropanamido) benzoic acid.
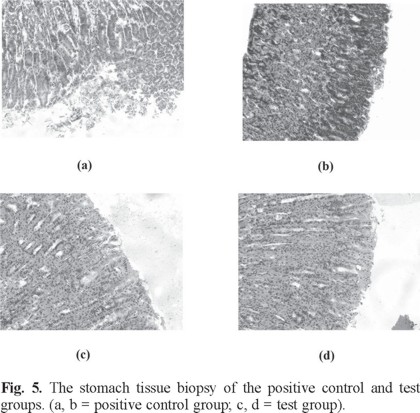
The anti-inflammatory, analgesic as well as the ulcerogenic activities of compound 1 was investigated in vivo at 100 mg/kg dosage and compared with aspirin as reference standard in all screenings. It is clearly seen from Fig. 5 that the positive control group (a and b) demonstrates ulcerogenic and bleeding points, versus test group (c and d); in addition, data showed in Table 2 indicated that compound 1 exhibits considerable anti-inflammatory and analgesic activities compared with aspirin, indicating that it is an active substance with anti-inflammatory and analgesic pharmacological activity.
Experimental
General experimental procedures
NMR spectra were recorded on Bruker AVANCE-600 NMR spectrometer, TMS as internal standard, δ in ppm, J in Hz. 13C multiplicities were determined by DEPT spectra. COSY, HMBC, HSQC and DEPT experiments were performed using Bruker microprograms. The optical rotation degree was measured PERKIN-ELMER241M optical rotation spectrometer, CD spectrum was recorded on Biologic MOS-450 CD spectrometer. The IR spectrum was recorded in the region 4000-400 cm-1 using KBr pellet technique with 1.0 cm-1 resolution on a BRUKER IFS-55V IR spectrometer. The FT-Raman spectra was recorded using 1064 nm line of Nd: YAG laser as the excitation wavelength in the region 3500-100 cm-1 on a BRUKER RFS 100/S FT-Raman spectrometer. The detector was liquid nitrogen cooled Ge detector. Two hundred scans were accumulated at 4 cm-1 resolution with 50mW laser power. Mass spectra were measured on Shimadzu 2010 LC-MS and a Bruker ESI-Q-TOF-MS/MS high-resolution mass spectrometer. Column chromatography was performed on silica gel (200-300 mesh; Qingdao Haiyang Chemicals).
Fungal materials
SYP-F-2720 is a sponge-derived marine fungus which belongs to Penicillium strains collected in 2003, Off the North Sea coast, China and deposited in School of Life Sciences, Shenyang Pharmaceutical University, China. The strain was maintained on Yeast extract-Malt extract Agar medium (0.09 g yeast extract powder, 0.09 g malt immersion powder, 0.15 g peptone, 0.3 g glucose, 0.45 g agar and 0.9 g NaCl in 30 mL distilled water) and cultivated at 28 °C for 5 days. Mycelial agar plugs were inoculated into a 2000 mL Erlenmeyer flask containing sterilized (glucose 1%, peptone 0.5%, yeast extract powder 0.3%, malt immersion powder 0.3%, NaCl 3% in 700 mL distilled water) at 28 °C for 14 days.
Extraction and Isolation
The fermentation broth of SYP-F-2720 was extracted by adding 1400 mL ethyl acetate (EtOAc) to the flask and then homogenized. The homogenized suspensions were collected, filtrated, and partitioned with water. Ethyl acetate portion was evaporated under reduced pressure to give brown syrup (0.1 g). Then the syrup was subjected to octadecylsilyl (ODS) column with linear gradient elution with 40%-100% aqueous methanol and 61 fractions were obtained. Fractions 1-4 were chromatographed with preparative-HPLC (MeOH-H2O 40 : 60) to produce compound 1 (1 mg).
Synthesic procedures of compound 1
Acetyl chloride (19.2 mL, 266.6 mmol) was added to the solution of L-Lactic (12.0 g, 133.3 mmol) and tetrahydrofuran (24 mL) at -5 °C, then the solution was stirred at room temperature for 5 h. The solution was evaporated under reduced pressure to obtain intermediate II which as colorless oily liquid (14.6 g, 83.6%). Then, the reaction mixture refluxed at 50 °C for 2h after added thionyl chloride (48 mL) to II (14.6 g, 110.6 mmol), and evaporated under reduced pressure to gain intermediate III which as light yellow oily liquid (15.6 g, 93.7%). Next, added the anhydrous ether solution of III (III: 15.6 g, 103.7 mmol, anhydrous ether: 25 mL) to the solution of methyl anthranilate (60.0 g, 395.8 mmol) and anhydrous ether (150 mL) at -5 °C, and stirred for 3 h, filtered. The filtrate was washed by HCl (100mL × 6, 1 mol/L), H2O (100 mL × 3) and NaCl (100 mL × 3), dried by anhydrous sodium sulphate, filtered and concentrated to acquire intermediate IV which as pale yellow crystalline (24.7 g, 89.9%). The sodium hydroxide solution (1 mol/L) was slowly dropped into the mixture of intermediate IV (12.7 g, 47.92 mmol), tetrahydrofuran (60 mL) and methanol (40 mL), and then stirred for 3h at room temperature. Next, the reaction solution was extracted with CH2Cl2 (200 mL × 3), and then adjusted the pH of the aqueous phase to 2 with HCl (1 mol/L), standing for crystallization, filtrated, and dried to afford (S)-2-(2-hydroxypropanamido)benzoic acid (8.79 g, 87.8%).
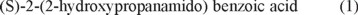
White needle crystals; [α] -15.6°(c, 0.10, MeOH); IR spectrum (KBr, v max, cm-1): 3400, 3267, 1689, 1660,1584, 1530, 1450, 1402, 1293, 1264, 1127, 876, 756; MS(ESI): m/z = 232.0582 [M + Na] ; 1H NMR (600 MHz, DMSO, δ): δH 13.55 (1H, s, NH), 11.99 (1H, s, COOH), 8.71 (d, J3,4 = 7.8 Hz, H-3), 8.01 (d, J5,6 = 7.8 Hz, H-6), 7.61 (dd, J3,4 = 7.8, J4,6 = 1.2 Hz, H-4), 7.17 (dd, J5,6= 7.8, J3,5 = 1.2 Hz, H-5), 6.13 (1H, s, 2'-OH), 4.16 (1H, m, H-2'), 1.34 (d, J = 6.6 Hz, CH3). 13C NMR (150 MHz, DMSO, δ): δc 174.4 (C-1'), 168.9 (COOH), 140.5 (C-2), 134.0 (C-4), 131.3 (C-6), 122.6 (C-5), 119.5 (C-3), 116.5 (C-1), 68.0 (C-2'), 20.9 (C-3'), see Table 1.
Computational details
The conformational searching for a pair of enantiomers (R and S) was performed by Spartan 10 program with MMFF molecular mechanics force field [19, 20]. After surveying the conformational space, the conformers within 5 kcal/mol enegy window were re-optimized at B3LYP level of theory using 6-311 + + G (2d, 2p) basis sets under PCM model by Gaussian 09 package program [21-23].
The Gibbs free energies were also calculated at the same level and frequency calculations based on the previously optimized geometries in order to ensure the minimum energy of the structure. Relative population of each conformer was valued on the basis of Boltzmann weighting factor at 298K to simulate ECD. The Boltzmann weighting factor
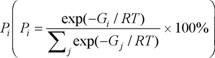 , where Gi, R and T stand for the Gribbs free energy, gas constant and absolute temperature (298 K).
, where Gi, R and T stand for the Gribbs free energy, gas constant and absolute temperature (298 K).
The ECD were simulated by the time-dependent density functional theory (TDDFT) method at CAM-B3LYP/TZVP and CAMB3LYP/aug-cc-pvdz basis set, and methanol was used as solution under PCM model. ECD curves were generated by Specdis with half bandwidth of 0.32 eV [16, 18, 19].
Biological activity assay
The anti-inflammatory and analgesic activities associated with the ulcerogenic assay of compound 1 were ascertained compared with aspirin which was used as positive control. The xylene-induced mouse ear edema model, acetic acid-induced writhing response model, and influence on the gastric irritation, following the reported method [24-29]. Pharmacological tests were conducted in Kunming mice weighing 20-25 g, and they were divided into nine groups of 10 mice each, of which three groups were all male mice for evaluating anti-inflammatory activity, three groups were all female mice for testing analgesic activity, the other three groups were either sex for evaluating ulcerogenic activity, pregnant female mice were excluded. Animal room was maintained at 25 ± 2 °C with a 12 hours light/dark cycle. Food and tap water was freely available except for the acute ulcerogenesis test. All test compounds were suspended in a solution of methylcellulose (0.5%) and administered orally at the dose of 100 mg/kg body weight. The results were summarized in Table 2. The ethical guidelines described in the NIH Guide for Care and Use of Laboratory Animals were followed throughout the experiments.
Conclusions
In summary, this paper reports the isolation and structural identification of (S)-2-(2-hydroxypropanamido) benzoic acid which was isolated from the fermentations of Penicillium chrysogenum SYP-F-2720. The structure with absolute configuration was determined by spectroscopic data coupled with quantum chemical calculations. Furthermore, the structural elucidation as well as AC was confirmed by the strong correlation of spectra between compound 1 and synthetic compound 1 which from raw material with a definite configuration. The anti-inflammatory and analgesic activities of the compound were evaluated and no ulcerogenic activity was observed simultaneously. Taken together, the results provide a new active substance which has anti-inflammatory and analgesic pharmacological activity.
Acknowledgements
This work was supported by the National Fund for Talent Training in Basic Science (No.J1103606), Program for Innovative Research Team of the Ministry of Education and Program for Liaoning Innovative Research Team in University. The theoretical calculations were conducted on the ScGrid and Deepcomp7000 the Supercomputing Center, Computer Network Information Center of Chinese Academy of Sciences.
References
1. Blunt, J. W.; Copp, B. R.; Munro, M. H. G.; Northcote, P. T.; Prinsep, M. R. Nat. Prod. Rep. 2006, 23, 26-78. [ Links ]
2. Donia, M.; Hamann, M. T. Lancet. 2003, 3, 338-348. [ Links ]
3. Bugni, T. S.; Ireland, C. M. Nat. Prod. Rep. 2004, 21, 143-163. [ Links ]
4. Saleem, M.; Ali, M. S. S.; Hussain, J. A.; Ashraf, M.; Lee, Y. S. Nat. Prod. Rep. 2007, 24, 1142-1152. [ Links ]
5. Venkateswarlu, Y.; Srinivasa, R. N.; Venkatesham, U. Chem Biomed Appl. 2001, 6, 101-128. [ Links ]
6. Boot, C. M; Amagata, T; Tenney, K; Compton, J. E; Pirtraszkiewicz, H; Valeriote. F. A; Crews. P. Tetrahedron. 2007, 63, 9903-9914. [ Links ]
7. Li, Q.; Wang, G. Microbiol Res, 2004, 52, 67-100. [ Links ]
8. Wu, B. W.; Hu, Y. H.; Fang, Z. J. Chin. Mar. Drugs. 2006, 25, 39-43. [ Links ]
9. Walter, C.; Christopher, N.; Catherine, H. J. Methods 2007, 42, 358-376. [ Links ]
10. Lin, W. H.; Fu, H. Z.; Li, J. Chin. Chem. Lett. 2001, 12, 235-238. [ Links ]
11. Gao, S. S.; Li, X. M.; Du, F. Y.; Li, C. S.; Proksch, P.; Wang, B. G. Drugs, 2011, 9, 59-70. [ Links ]
12. Bugni, T. S.; and Ireland, C. Nat. Prod. Rep. 2004, 21, 143-163. [ Links ]
13. Wang, J. J.; Zhao, Y. L.; Men, L.; Zhang, Y. X.; Liu, Z.; Sun, T. M.; Geng, Y. D.; Yu, Z. G. Chem. Nat. Compd. 2014, 50, 405-407. [ Links ]
14. Yu, Z.G; Zhao, Y.L; Wang, J.J. Chian patent, 201210541957.8, 2014. (CN 103864638 A). [ Links ]
15. Guan, J.; Zhao, Y. L.; Zhu, H. Y.; An, Z. Z.; Yu, Y. M.; Li, R. J.; Yu, Z. G. J. Pharm. Biomed. 2014, 95, 20-25. [ Links ]
16. Harada, N.; Nakanishi, K.; Circular dichroic spectroscopy: exciton coupling in organic stereochemistry, University Science Books, Mill Valley, 1983. [ Links ]
17. Giuseppe Mazzeo, G.; Santoro, E.; Andol, A.; Cimmino, A.; Troselj, P.; Petrovic, A. G.; Superchi, S.; Evidente, A.; Berova, N. J. Nat. Prod. 2013, 76, 588-599. [ Links ]
18. Miertus, S.; Tomasi, J.; J. Chem. Phys. 1982, 65, 239-245. [ Links ]
19. Spartan 08, Wavefunction Inc., Irvine, CA 92612, USA, 2008. [ Links ]
20. Berova, N.; Nakanishi, K.; Woody R. W. Circular dichroism: Principles and Applications, 2nd ed.; Wiley-VCH, NewYork, USA, 2000. [ Links ]
21. Miertus, S.; Scrocc, E.; Tomasi, J. J. Chem. Phys. 1982, 55, 117-129. [ Links ]
22. Evidente, A.; Superchi, S.; Cimmino, A.; Mazzeo, G.; Mugnai, L.; Rubiales, D.; Andol, A. Eur. J. Org. Chem. 2011, 5564-5570. [ Links ]
23. Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, M. J.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J.; Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford CT, 2010. [ Links ]
24. Romay, C.; Armesto, J.; Remirez, D.; Gonzalez, R.; Ledon, R.; Garcia, N. J. Inflamm. Res. 1998, 47, 334-348. [ Links ]
25. Gu, K.; Bi, L.; Zhao, M.; Wang, C.; Kao, M.; Tok, J.; Peng, S. Bioorg. Med. Chem. 2006, 14, 1339-1347. [ Links ]
26. Bi, L.; Zhang, Y.; Wang, M.; Tok, C.; Peng, J. Bioorg. Med. Chem. 2005, 13, 5640-5646. [ Links ]
27. Siegmund, E. A.; Cadmus, R. A.; Lu, G. Proc. Soc. Exp. Biol. Med. 1957, 95, 729-731. [ Links ]
28. Verma, M.; Sinha, J. N.; Gujrati, V. R. Pharmacol. Res. Commun.1981, 13, 967-979. [ Links ]
29. Suleyman, H.; Akcay, F.; Altinkaynak, K. Pharmacol. Res. 2002, 45, 155-158. [ Links ]














