Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.58 no.3 Ciudad de México jul./sep. 2014
Article
Removal of Color and Chemical Oxygen Demand Using a Coupled Coagulation-Electrocoagulation-Ozone Treatment of Industrial Wastewater that Contains Offset Printing Dyes
Gabriela Roa-Morales,1,* Carlos Barrera-Díaz,1 Patricia Balderas-Hernández,1 Francisco Zaldumbide-Ortiz,1 Horacio Reyes Perez,2 Bryan Bilyeu3
1 Centro Conjunto de Investigación en Química Sustentable UAEM-UNAM. Km 14.5 Carretera Toluca-Atlacomulco, Campus UAEMex "El Rosedal" San Cayetano-Toluca, C.P. 50200, Estado de México, México. Tel: 722-2766610. groam@uaemex.mx
2 Universidad Autónoma del Estado de México. Facultad de Química. Paseo Colón intersección Paseo Tollocan S/N. C.P. 50120, Toluca, Estado de México, México.
3 Xavier University of Louisiana, Department of Chemistry, 1 Drexel Drive, New Orleans, LA 70125, USA.
Received January 31st, 2014
Accepted May 8th, 2014.
Abstract
Industrial offset printing processes generate wastewater with highly colored obtaining values of 5x106 Pt-Co units and great values of chemical oxygen demand (COD) 5.3x10−5 mg L−1. Thus, conventional technologies such as biologicals treatment fail in reaching the discharge limits. In this research, a sequential treatment was applied: coagulation with aluminum hydroxychloride (AHC), electrocoagulation with Al anodes and finally ozonation. Optimal conditions are found when adding 20 mg L−1 AHC, followed by electrocoagulation at 4 A for 50 min, and finally alkaline ozonation for 15 min, resulting in an overall color removal of 99.99% color and 99.35% COD.
Key words: Dye, AHC, Coagulation, Electrocoagulation, Ozonation.
Resumen
El agua residual generada por la industria de la impresión de tinta contiene una alta demanda química de oxígeno (DQO) correspondiente a 5.3x10−5 mgL−1 así como una alta coloración que presenta una medición de 5x106 en unidades Pt-Co. Cuando se aplican métodos biológicos de tratamiento, presentan bajas eficiencias por lo que no se alcanzan los límites máximos permisibles de descarga. En esta investigación se aplicó un tratamiento secuencial: coagulación con hidroxicloruro de aluminio (HCA), electrocoagulación con ánodos de Al y ozono a agua residual proveniente de la industria de impresiones. Los resultados más eficientes correspondían a la coagulación con HCA a una dosis de 20 mg L−1, electrocoagulación a 4 A durante 50 min, y la ozonización alcalina durante 15 min, dando como resultado remoción total de 99,99% de color y 99,35% de DQO.
Palabras clave: colorantes, HCA, coagulación, electrocoagulación, ozonación.
Introduction
One million tons of more than ten thousand types of synthetic dyes and pigments are produced annually worldwide and are used extensively in many diverse industrial fields, such as textile and leather industries, paper production, food processing, agricultural research, light-harvesting arrays, photo-electrochemical cells, and hair coloring [1-3].
Traditional methods for treating textile wastewater use various combinations of biological, physical, and chemical methods [4, 5]. However, common biological treatment processes are often ineffective in removing dyes since these chemicals contain highly structured polymers with low biodegradability [6].
Electrochemical technology contributes in many ways to a cleaner environment and covers a very broad range of technology [7]. The generation of energy using new batteries [8], fuel cells [9], selective synthesis of organic chemicals [10], recycling of process streams and wastewater treatment are the most common [11].
During the last two decades, a new research field has been developed, namely environmental electrochemistry. Environmental electrochemistry involves electrochemical techniques or methods to remove impurities from gases, liquids and soil to prevent or minimize environmental pollution [12-14].
Electrocoagulation has been proposed as an effective method to treat various wastewaters [5] from leachate [15], restaurants [16], saline [17], olive oil [18], urban sources [19], laundry [20], nitrate and arsenic by products [21, 22], and chemical mechanical polishing [23].
In essence an electrocoagulation reactor is an electrochemical cell wherein a sacrificial metal anode, usually aluminum but occasionally iron, is used to dose polluted water with a metal ion coagulating agent following reactions (1-3) [24, 25].
Anode Fe → Fe2+ + 2e− (1)
Cathode H2O + 2e− → H2 + OH− (2)
In solution Fe2+ + 2OH− → Fe(OH)2 (3)
The use of Al as the sacrificial electrode has been mainly applied as an electrocoagulation technique in the removal of dyes, pollutants in surface waters and clay in potable water, among others [26-28]. The formation of Al coagulant is shown in reactions (4-6).
Anode Al → Al3+ + 3e− (4)
Cathode H2O + 2e− → H2 + OH− (5)
In solution Al3+ + 3OH− → Al(OH)3 (6)
Compared with conventional water treatment processes, in which chemicals are added to water to cause precipitation and enhance flocculation, electrocoagulation has several advantages including reduction of waste volume, lower cost, and improvised solid-liquid separation [29].
On the other hand, ozonation is one of the most attractive alternatives for solving the problem of color in textile effluents. Ozone is an extremely strong oxidant (E˚ = 2.07 V) and reacts rapidly with most organic compounds. Ozone is selective and preferentially attacks the unsaturated bonds of chromophores, which are often associated with color [30].
Ozone reacts with water and wastewater compounds in two different ways, namely direct molecular and indirect free radical-type reactions. Both pathways are important for color removal. Application of ozone to decolorize different classes of textile dyes is a well-known process and has been used for two decades [31].
Ozonation rarely produces complete mineralization to carbon dioxide and water, but leads to partial oxidation sub-products such as organic acids, aldehydes and ketones [30]. The mineralization of organic compounds strongly depend on the oxidation conditions.
Ozonation is regularly used as a pre-oxidation step for organic matter prior to coagulation (followed by filtration) and/or as a final purification/disinfection step, during the treatment of surface waters for the production of drinking water [32].
In this study we present the results of a combined coagulation-electrochemical and ozone treatment into an integrated process as a promising alternative for the treatment of wastewater containing compounds with high color values and low biodegradability.
Experimental
Wastewater samples
Samples of wastewater were collected at the outlet of an industrial process which uses offset dyes to print cardboard boxes. The wastewater has different colors (blue, green, black and red), depending on the printing jobs done. This wastewater contained is highly turbid.
The daily volume of residual wastewater was 12 m3. The samples were collect in plastic containers and cooled to 4 ºC, then transported to the laboratory for analysis and treatments.
Coagulation
In coagulation experiments Al2(SO4)3 analytical grade (Fermont) and aluminum hydroxychloride (AHC) from Clariant® 90% of purity were used as coagulants, added to the aqueous dye solutions at a volume of 0.5 L. The pH of the dye sample was not adjusted. The jar test was used to perform these experiments under the following conditions: 2-3 min rapid mixing at 180-190 rpm, followed by 10 min of slow mixing at 30 rpm. Afterward, samples were filtered through filter paper.
Electrochemical reactor and electrocoagulation
A batch electrochemical reactor was constructed for the electrocoagulation step. The electrode system is monopolar. Fig. 1 shows a schematic diagram of the electrochemical reactor.
The reactor cell contains an array of 8 parallel Al electrodes. Each electrode is 0.15 m long by 0.1 m wide, with a surface area of 0.03 m2, for a total anodic surface, Aa of 0.12 m2. The distance between the electrodes was of 2 cm. Each 2.5 dm3 batch of water was treated in a bucket which served as the supply vessel for the reactor. A study of variation of the current density was conducted to evaluate the best conditions for the electrocoagulation experimental applying 15.15, 33.33, 45.45 and 66.6 A m−2 using DC power source.
Ozonation
Previously treated wastewater was used in the ozone reactor. Ozonation experiments were carried out in a 0.5 L Pyrex reactor with a continuous 5 g h−1 supply of O3. Ozone was generated by means of a Pacific Ozone Technology instrument using air as a feeding gas. The ozone-air mixture was fed through diffusers placed at the bottom of the reactor. Ozone gas concentration in the influent and effluent gas was measured by the Potassium Iodide Method [33]. The gaseous outlet from the reactor was led to a killer, where the remaining ozone was destroyed by means of a reaction with KI. All experimental procedures were realized by duplicate.
Methods of analysis
The initial evaluation of the electrochemical and ozone treatments was determined by analysis of the COD, Color (Pt/Co unit), pH, and turbidity at different time intervals, as indicated in the standard methods procedures [33].
Cyclic Voltammetric measurements
Cyclic voltammetry of crude and treated wastewater was performed using a standard three-electrode cell. The waveforms were generated by a potentiostat model BAS-100W, controlled by BAS software. The carbon paste electrodes (CPE) were circular with a surface area of about 5 mm2. The CPE was prepared from a 1:1 mixture of 99.99% pure single crystal graphite (Alfa AESAR) and nujol oil (Fluka). The paste was transferred into a PVC tube and compacted to eliminated trapped air then a copper conductor was inserted before the paste set. The surface of the electrode was renovated after each potential scan. The scan rate was 100 mV s−1. The reference electrode was an Ag/AgCl saturated with KCl and the counter electrode was a platinum wire.
UV- Vis spectrometry
UV-Vis spectra were obtained from samples of raw and treated wastewater using a double beam Perkin-Elmer 25 spectrophotometer. The scan rate was 960 nm s−1 within the 200-900 nm wavelength range. The samples were scanned in quartz cells with a 1 cm optical path.
Sludge Characterization
The sludge generated by the coagulation process was analyzed by scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) microanalysis. The analysis was performed on a Philips XL-30 microscope to observe the composition and configuration of the structure. SEM provides images of material with resolution down to fractions of a micrometer, while EDX offers in situ elemental analysis.
Results and discussion
Wastewater characteristics
The offset printing process uses a wide range of dyes. The most common colors are black, red, blue, yellow and green. However, the red removal requires the strongest conditions of all. Therefore, the following conditions correspond to the red treatment which ensures that all the dyes producing color in wastewater can be eliminated.
Because the wastewater contains a high concentration of suspended solids, using direct electrocoagulation makes that electrodes passivate due to the accumulation of the suspended particles, so it was decided to remove them previously by chemical coagulation as shown below.
Coagulation
Coagulation experiments were carried out using the jar test. The optimal AHC and Al2(SO4)3 doses are shown in Tables 1 and 2. In Table 1 the initial dye concentration in the residual wastewater is high, but increasing the coagulant dose reduces the COD, color and turbidity. Table 1 shows that using Al2(SO4)3 is not very effective since small pollutant reductions are reached with additions as high as 500 mgL−1. Indeed, a huge dose of 8500 mg L−1 is required to achieve an 83% reduction in COD.
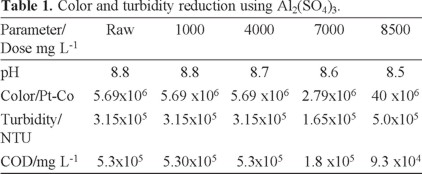
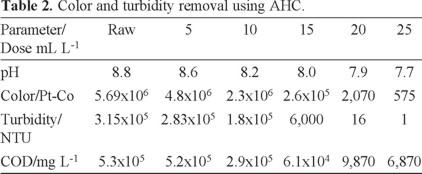
It is interesting to observe in Table 2 that a high reduction of the COD is attained when AHC is added to wastewater. However, beyond 20 mL of AHC the reduction is less dramatic. Therefore, an optimum dose of 20 mL AHC was selected for further treatment.
AHC is a pre-polymerized Al(III) compound, containing a range of hydrolysis and polymeric species which are relatively large and carry a high cationic charge. Their enhanced surface activity and improved charge neutralizing capacity may make them more effective at a comparatively lower dose than aluminum sulfate. Our results agree with other reports that indicate that compared to conventional Al(III) salts, AHC has the following advantages; rapid aggregation velocity, larger and heavier flocs and lower required dosage [5, 34].
Electrocoagulation
Wastewater samples were taken from the previous coagulation experiments with a COD of 9870 mgL−1, then electrochemically treated using the reactor described earlier, adjusting the pH (using NaOH or H2SO4) and applying 4 A of current.
Current density not only determines the coagulant dosage rate, but also the bubble production rate and the floc growth, which can influence the treatment efficiency of the electrocoagulation. Therefore, the effect of current efficiency on the removal of pollutants was investigated.
Figure 2 shows the variation of the COD as a function of current density. At 50 min of treatment time, the three highest current densities produce similar COD reductions: 33.3 A m−2 (61.27%), 45.45 A m−2 (61.71%), and 60.6 A m−2 (62.3%). Moreover, higher current densities have been found to increase the need for maintenance and cleaning of the electrodes, so current densities around 20-25 A m−2 are recommended from a maintenance standpoint [35].
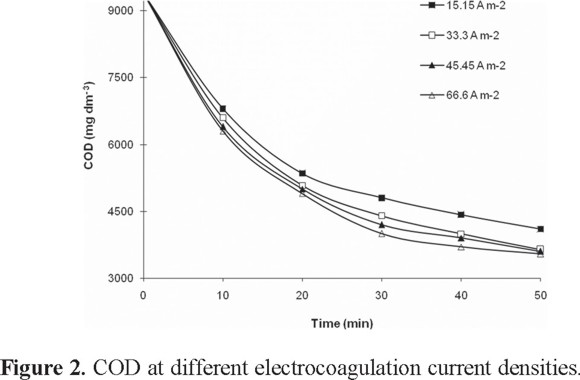
The best experimental conditions for the electrocoagulation treatment were at pH 8 and 33.3 A m−2 of current density. Table 3 shows the COD, color, and turbidity reduction as a function of treatment time under these conditions.
The COD reduction as a function of time is show in Fig. 2.
After 50 min of treatment, reductions of 62% of COD, 55% of color, and 81% of turbidity were obtained. These values are consistent with data reported in the literature in which the use of electrocoagulation with aluminum electrodes in wastewater from a plant paste processor, results in near COD removal of 64% at pH 8 during 45 min of treatment applying 18.2 mA m−2 [28]. Another study indicates that contamination of textile wastewater can be made using electrocoagulation with aluminum electrodes and 100 A m−2 of current density during 10 min, producing a reduction of 50% COD [5]. The discoloration of the dye Remazol red RB 123 by means of electrocoagulation with aluminum electrodes to pH 6, obtaining 100% color removal when the initial concentration of the dye was 100 mg L−1 and 70% color removal when the initial concentration of the dye was 1000 mgL−1 after 10 min of a 100 A m−2 current density. These investigators conclude that the greater the initial concentration of dyes, the smaller the reduction in color [4]. Finally, electrocoagulation with aluminum electrodes for discoloration and removal of phenolic compounds and observed that the efficiency in the color removal during 30 min of treatment at pH 8 was 82%, and with respect to the COD, an efficiency of 52% is had using 300 A m−2 of current density [36].
The COD reduction kinetics was evaluated using the first and second order equations through a final electrolysis time of 50 minutes taking the data of Fig. 2 with the conditions of pH 8 and 33.3 A m−2. The value for the first order rate constant is k1 = 0.019 min−1 with R2 = 0.9105; however the second order model gives R2= 0.9622 and k2 = 3x10−6 dm3 mg−1min−1. The second order model (equation 7) describing the decrease in COD with time is:
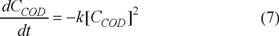
Where CCOD represents the [COD] mg dm−3, t represents the time, and k the rate constant in dm3 mg−1 min−1.
The kinetics of the electrocoagulation COD reduction is shown in Figure 3
Recent research supports second order kinetics for the COD reduction for electrocoagulation treatment [37, 38].
Ozonation
The previous coagulated/electrocoagulated sample was used with the parameters that are given in the first column of Table 4 and the ozonation was applied under those conditions.
The ozone treatment had little effect on COD or pH of wastewater. However, there is a significant color reduction; indeed, the satisfactory time for the application of this treatment is 15 min.
The efficiencies obtained in this investigation by means of ozonation were 90% of color removal and nearly 9% of COD reduction during 15 min of treatment. Another study in wastewater that contains dyes originating from the textile industry use oxidation by ozone during 15 min of treatment and obtains efficiencies of 92% of color removal to a wavelength of 436 nm, 96.8% to a wavelength of 525 nm, and 100% to a wavelength of 620 nm [39]. With respect to the COD (initial COD 1340 mg L−1), they show efficiencies of 12% of removal in 15 min and 16% if the ozonation is extended to 30 min. Furthermore, bleach industrial wastewater containing dyes can be treated with efficiencies of 92% for the color removal but only 7.85% for COD reduction [40].
UV-Vis spectra
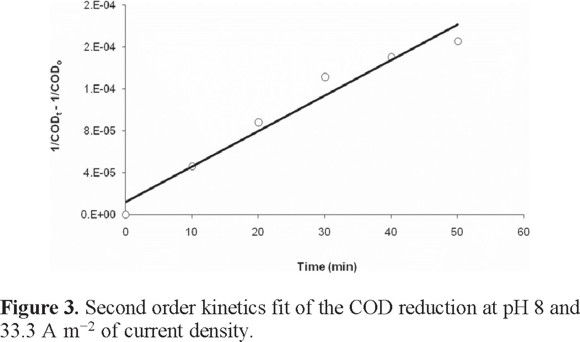
The UV-Vis spectra of the raw, coagulated, electrochemically treated and ozonated wastewater are shown in Fig. 4. There is a continuous signal curve in the region of 200-900 nm in the spectra corresponding to components of the wastewater. It is interesting that the intensity of the curves decreases as coagulation, electrochemical and ozone treatments are applied. The peak around 550 nm decreases by 99% when chemical treatment is applied [absorbance decreases from 10 to 0.9). In the region of 200 nm the reduction are 15, 75 and 85% respectively for the coagulation, electrocoagulation and ozonation. These results indicate that there is a significant color reduction of the raw wastewater when the treatments are applied.
Cyclic voltammetry
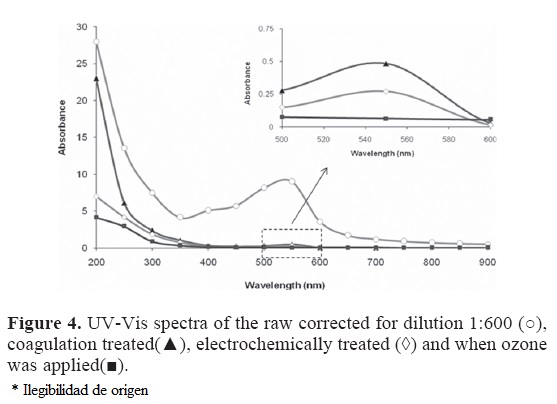
To determine the electrochemical characteristics of the raw and treated wastewater, and the processes occurring at the electrodes, a series of cyclic voltammetry experiments were performed using a CPE as the working electrode. Cyclic voltammetry results indicate that a chemically irreversible oxidation peak in the wastewater is detectable at potentials lower that those corresponding to oxygen evolution as shown in Fig. 5. This peak corresponds to the direct electrochemical oxidation of pollutants present in wastewater. It is important to note that when cyclic voltammetry is applied after wastewater treatments, the peak reduces and finally does not appear in the voltammogram indicating that pollutants in the solution have been oxidized. Thus, these voltammograms clearly indicate that there are processes attributable to elimination of pollutants.
Scanning electron microscopy microphotographs
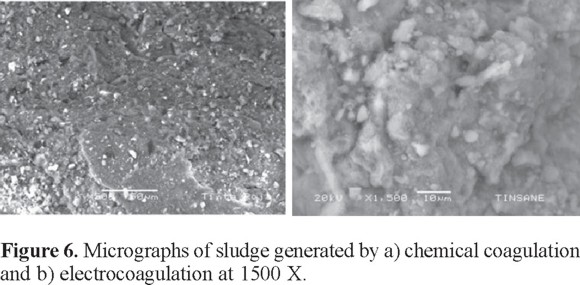
Previous studies indicate that the morphology and elemental composition of sludge can be determined by SEM and energy dispersive x-ray spectroscopy (EDS). The hollows or cavities in the structure of sludge can be seen in the SEM images, whereas EDS identifies the elemental composition and the relative amounts of elements present in the sludge [41, 42]. SEM images of the sludge generated by coagulation and by electrocoagulation were collected to compare the morphologies. The micrograph in Fig. 6(a) corresponds to the sludge from the coagulation process showing shiny sharp crystals. Fig. 6(b) shows the sludge from electrocoagulation with more rounded compact agglomerates.
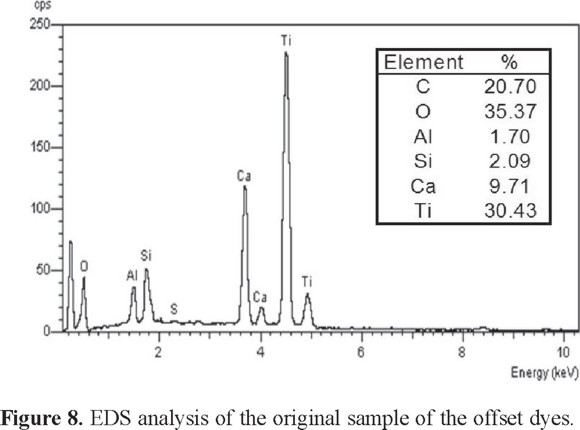
Analysis of the X-rays generated by SEM using EDS allows elemental analysis of the sample, as shown in Fig. 7. The chemically coagulated (Fig. 7a) and electrocoagulated (Fig. 7b) sludge both have C, O, Al, Ca, S, and Cl, although the relative compositions are different. The chemically coagulated sludge has a higher percentage of carbon (57%) than the electrocoagulated one (9%), perhaps indicating an advantage in precipitating organic components. The electrocoagulated sludge had a higher percentage of aluminum (33%) and oxygen (48%) than the chemically coagulated content (4 and 30% respectively) as expected due to the formation of aluminum hydroxide. It is observed in addition, that the coagulated sample indicates the presence of Ti because this element is one of the multiple components that contain the offset dyes (Fig. 8).
Conclusions
AHC is effective as a pre-treatment for the electrochemical process since it contributes to a large reduction in COD, color, and turbidity with a small amount of coagulant. Electrocoagulation of the pre-treated wastewater further reduces the COD, turbidity and color by a different method with likely different selectivity. Ozone eliminates the remaining color, but has little effect on COD, so is used as a final polishing step for color. The combined process presents accumulated efficiencies of 99.99% in color removal and significantly reduces the concentration of organic polluting agents in the wastewater. The UV-Vis spectrometry and the cyclic voltammetry confirm the increase in the quality of the water.
Acknowledgments
The authors wish to acknowledge the support given by the Universidad Autónoma del Estado de México specifically the Facultad de Química (Project UAEM 2452, PROMEP 123 and CONACYT 2838).
References
1. Rodriguez, M.; Sarria, V.; Esplugas, S.; Pulgarin, C. J. Photochem. Photobiol. A. 2002, 151, 129-35. [ Links ]
2. Forgacs, E.; Cserháti. T.; Oros, G. Environ. Int. 2004, 30, 953-971. [ Links ]
3. Kulik, N.; Panova, Y.; Trapido, M. Separ. Sci. Technol. 2007,42, 1521-1534. [ Links ]
4. Can, O.T.; Bayramoglu, M.; Kobya, M. Ind. Eng. Chem. Res. 2003, 42, 3391-3396. [ Links ]
5. Can, O.T.; Kobya, M.; Demirbas, E.; Bayramoglu, M. Chemosphere 2006, 62, 181-187. [ Links ]
6. Lin, S.H.; Chen, M.L. Water Res. 1997, 31, 868-876. [ Links ]
7. Janssen, L. J. J.; Koene, L. Chem. Eng. J. 2002, 85, 137-146. [ Links ]
8. Vincent CA. Modern batteries, An introduction to electrochemical power sources. Ed. Elsevier. 1984. [ Links ]
9. Kordesch, K.; Simader, G. Fuel Cells. Their Applications. VCH, New York. 1996. [ Links ]
10. Utley, J. Chem. Soc. Rev. 1997, 26, 157-167. [ Links ]
11. Cushnie, Jr. G. C. Electroplating wastewater pollution control technology. Ed. Noyes Publications . U.S.A. 1985. [ Links ]
12. Rajeshwar, K.; Ibanez, J. G. Environmental electrochemistry: Fundamentals and applications in pollution sensors and abatement. Ed. Academic Press Limited. U.S.A. 1997. [ Links ]
13. Jüttner, K.; Galla, U.; Schmieder, H. Electrochim. Acta. 2000, 45, 2575-2594. [ Links ]
14. Simonsson, D. Chem. Soc. Rev. 1997, 26, 181-189. [ Links ]
15. Ilhan, F,; Kurt, U.; Apaydin, O.; Gonullu, M. T. J. Haz. Mat. 2008, 154, 381-389. [ Links ]
16. Chen, X.; Chen, G.; Yue, P. L. Sep. Purif. Technol. 2000, 19, 65-76. [ Links ]
17. Lin SH, Shyu CT, Sun MC. Water Res. 1998, 32, 1059-66. [ Links ]
18. Inan, H.; Dimoglo, A.; Simsek, H.; Karpuzcu, M. Sep. Purif. Technol. 2004, 36, 23-31. [ Links ]
19. Pouet, M.F.; Grasmick, A. Water Sci Technol. 1995, 31, 275-83. [ Links ]
20. Ge, J.; Qu, J.; Lei, P.; Liu, H. Sep. Purif. Technol. 2004, 36, 33-39. [ Links ]
21. Koparal, A. S.; Ogutveren, U. B. J. Haz. Mat. 2002, 89, 83-94. [ Links ]
22. Ratna, K. P.M.; Chaudhari, S.; Khilar, K. C.; Mahajan, S. P. Chemosphere 2004, 55, 1245-1252. [ Links ]
23. Lai, C. L.; Lin, S. H. Chemosphere 2004, 54, 235-242. [ Links ]
24. Vik, E. A.; Carlson, D. A.; Eikum, A. S.; Gjessing, E. T.. Water Res. 1984, 18, 1355-1360. [ Links ]
25. Holt, P. K.; Barton, G. W.; Mitchell, C. A. Chemosphere 2005, 59, 355-367. [ Links ]
26. Jiang, J. Q.; Graham, N. André, C.; Kelsall, G. H.; Brandon, N. Water Res. 2002, 36, 4064-4078. [ Links ]
27. Holt, P. K.; Barton G. W. Water Sci. Technol. 2004, 50, 177-184. [ Links ]
28. Roa, M. G.; Campos, M. E.; Aguilera, C. J.; Bilyeu, B.; Barrera, D. C. Sep. Purif. Technol. 2007, 54, 124-129. [ Links ]
29. Tsouris, C.; DePaoli, D.W.; Shor, J. T.; Hu, M.Z.C., Ying, T.Y. Colloid. Surface A. 2000, 177, 223-233. [ Links ]
30. Soares, O.S.G.P.; Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Separ. Sci. Technol. 2007, 42, 1477-92. [ Links ]
31. Swaminathan, M,; Muruganandham, M.; Sillanpaa M. Int. J. Photoenergy 2013, 1-3. [ Links ]
32. Gottschalk, C.; Libra, J. A.; Saupe, A. Ozonation of water and waste water: A practical guide to understanding ozone and its applications. John Wiley & Sons. 2009. [ Links ]
33. Standard Methods for the Examination of Water and Wastewater Procedures (APHA, AWWA and WPCF), 1989. [ Links ]
34. Gao, B.; Yue, Q.; Wang, B. J. Environ. Sci. Heal. A. 2003, 38, 897-907. [ Links ]
35. Chen, G. Sep. Purif. Technol. 2004, 38, 11-41. [ Links ]
36. Adhoum, N.; Monser, L. Chem. Eng. Process. 2004, 43, 1281-1287. [ Links ]
37. Kobya, M.; Hiz, H.; Senturk, E.; Aydiner, C.; Demirbas, E. Desalination 2006, 190, 201-211. [ Links ]
38. Aleboyeh, A.; Daneshvar, N.; Kasiri, M. B. Chem. Eng. Process. 2008, 47, 827-832. [ Links ]
39. Doruel, S.; Dulekgurgen, E.; Orhon, D. J. Chem. Technol. Biot. 2006, 81, 426-432. [ Links ]
40. Hassan, M.M.; Hawkyard, C. J.; Barratt, P. A. J. Chem. Technol. Biot. 2006, 81, 158-166. [ Links ]
41. Baron, D.; Palmer, C. D.; Stanley, J. T. Environ. Sci. Technol. 1996, 30, 964-968. [ Links ]
42. Mahmood, T.; Zawadzki, M.; Banerjee. S. Environ. Sci. Technol. 1998, 32,1813-1816. [ Links ]














