Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.58 no.3 Ciudad de México jul./sep. 2014
Article
Use of Combined Electrochemical Approaches for Mineralization and Detection of Hydroquinone Using PbO2 Electrodes
Alexsandro Jhones dos Santos,1 Daniela Karla de Souza Xavier,1 Djalma Ribeiro da Silva,1 Marco Antonio Quiroz,2 and Carlos A. Martínez-Huitle1,*
1 Federal University of Rio Grande do Norte, Institute of Chemistry, Lagoa Nova CEP 59078-970 - Natal, RN, Brazil. carlosmh@quimica.ufrn.br, Tel/Fax.: +55 (84) 3211-9224.
2 Universidad de las Américas Puebla. Grupo de Investigación en Energía y Ambiente. ExHda. Sta. Catarina Mártir s/n, Cholula 72820, Puebla, México.
Received February 12th, 2014
Accepted April 29th, 2014.
Abstract
The electrochemical oxidation (EO) of hydroquinone (H2Q) has been performed, in acidic media, at PbO2 electrode by galvanostatic electrolysis applying 10 and 30 mAcm−2. The concentration of H2Q during its EO was also monitored by differential pulse voltammetry (DPV) by using PbO2 electrode. The experimental results of galvanostatic electrolyses showed that the performances of the process remarkably depend on the applied current density and in particular, the removal efficiencies obtained at PbO2 anode were 100% and 80%, at 30 and 10 mAcm−2, respectively. Additionally, the electroanalytical technique was efficiently used as a detection method during H2Q electrooxidation and when DPV analyses were compared with HPLC method, it achieved a good fit, confidence intervals and limits.
Keywords: hydroquinone, electrochemical oxidation, differential pulse voltammetry, electroanalysis.
Resumen
La oxidación electroquímica (OE) de hidroquinona (H2Q) se llevó a cabo en medio acuoso ácido, en el electrodo PbO2 por electrólisis galvanostática, aplicando 10 y 30 de mAcm−2. La concentración de H2Q durante su EO fue también monitoreada por voltametría diferencial de pulso (VDP) mediante el uso del electrodo PbO2. Los resultados experimentales de las electrólisis galvanostáticas mostraron que la eficiencia del proceso dependen de manera notable de la densidad de corriente aplicada y, en particular, las eficiencias de eliminación obtenidas usando el ánodo de PbO2 fueron 100% y 80% a las densidades de corriente de 30 y 10 mAcm−2, respectivamente. Adicionalmente, la técnica electroanalítica fue eficientemente usada como un método de detección durante la oxidación electroquímica de H2Q y cuando los análisis efectuados por VDP fueron comparados con el método CLAR, lograron un buen ajuste, intervalos de confianza y límites.
Palabras clave: hidroquinona, oxidación electroquímica, voltametria de pulso diferencial, electroanálisis.
Introduction
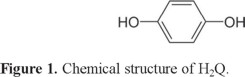
Hydroquinone (1,4-dihydroxybenzene, H2Q) is an isomer of phenolic compounds (Figure 1), which are considered as environmental pollutants by the US Environmental Protection Agency (EPA) and the European Union (EU) [1]. It is widely used in cosmetics, tanning, pesticides, flavoring agents, medicines, and photography chemicals [2] and can easily be introduced into the environment as pollutants. High concentration of H2Q can lead to fatigue, headache, and tachycardia to human body and also can damage kidney [3, 4]. Because of its coexistence in environmental samples; efficient, versatile as well as highly sensitive and selective, methods are strongly demanded for the elimination and determination of H2Q.
In this context, electrochemical technologies have a continuous growing importance in the field of decontamination of effluents [5-8] as well as detection of pollutants. In the former, several anodic materials [6,7], such as boron-doped diamond (BDD) [9-11], lead dioxide [12], tin dioxide [13, 14], platinum, graphite, ruthenium oxide, iridium oxide, etc., have been tested for the destruction of a large variety of model and real wastewaters [6, 7]. However, for the electrochemical approaches for abatement of pollutants, anodes with high oxygen evolution overpotential (i.e. anodes that are poor catalysts for oxygen evolution reaction), such as antimony-doped tin oxide, boron-doped diamond (BDD), or lead dioxide [13-19], favor complete oxidation of the organics to CO2. Therefore, they are ideal electrodes for wastewater treatment.
For detection analysis, chromatographic and optical methods are preferable over electrochemical methods for detecting and quantifying the pollutants concentration remaining in solution or in the effluent. However, these methods have some drawbacks such as high cost, low sensitivity, insufficient selectivity and long analysis times. Instead, electrochemical methods satisfy many of the requirements for such tasks particularly owing to their inherent specificity, speed of response, more feasible for miniaturization of analysis; sensitivity and simplicity of preparation respect other techniques as chromatographic and spectrophotometric.
Based on this information, we prepared a PbO2 electrode and this sensor was applied for the quantification of H2Q during its electrochemical oxidation at Pb/PbO2 anode in order to demonstrate the applicability of the combined electrochemical technologies for environmental applications as well as its great response respect to chromatographic assays.
Experimental
Reagents
Chemicals were of analytical grade, and all solutions were prepared with MilliQ water. Two solutions of H2Q were prepared in 0.5 mol L−1 H2SO4, using MilliQ water: (i) 200 mg L−1 (used for electroanalysis procedures as standard solution) and (ii) 50 mg L−1 (used for electrochemical oxidation experiments).
Preparation of PbO2 electrode
The initial, geometric surface area of the sheet of pure lead, used to prepare the working electrode, was approximately 20 cm2. After a deep cleaning of the exposed lead surface [20], the latter was anodized at a current density of 50 mAcm−2 during an electrolysis time of 1.5 h in a 10% H2SO4 solution at 25 °C, in order to oxidize the lead surface into PbO2 [20-22]:
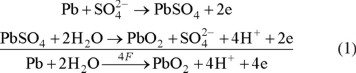
Surface Analysis
The surface morphology of PbO2 electrode was investigated by scanning electron microscopy (SEM). These images were performed with SEM PHILLIPS XL-30 - ESEM with Sputter Coater BAL-TEC Model SCD-005.
Analytical methods
Polarization curve measurements were performed using Autolab model PGSTAT320. Pb/PbO2 was used as working electrode (0.2 cm2 of exposed area), Ag/AgCl electrode and Pt wire were used as reference and counter electrodes, respectively, in H2SO4 0.5 mol L−1.
During the EO tests, the H2Q concentration of collected samples was determined by differential pulse voltammetry (DPV) with calibration curve. Electrochemical analyses were carried out by using Autolab model PGSTAT320. DPV measurements were usually conducted in the potential window from 0.0 to 1.2 V in H2SO4 0.5 mol L−1. The experiments were carried out at 25°C with a conventional three-electrode system, and applying scan rate of 50 mV s−1; equilibration time of 10 s; modulation time of 0.04 s; initial potential of 0.0 V; end potential of 1.2 V; step potential of 0.006 V; modulation amplitude of 0.05 V and standby potential of 0.05 V. A PbO2 electrode with an exposed geometric area of ca. 0.8 mm2 was used as the working electrode. A platinum wire and an Ag/AgCl (3 mol L−1 KCl) were employed as the auxiliary and reference electrodes, respectively. Calibration curves for H2Q were obtained in 0.5 mol L−1 of H2SO4, evaluating the peak intensity as a function of the analyte concentration, and considering at least sixteen analyte concentrations. Every experiment was performed by using a newly prepared electrode, in 30 mL of supporting electrolyte, after that, by adding known volumes of the H2Q solution of 200 mg L−1 in the measuring vessel.
Electrochemical oxidation experiments
Bulk oxidations were performed in undivided electrochemical cell, the reaction compartment having a capacity of 0.4 L, and the solution was stirred by a magnetic stirrer. The experiments of EO of H2Q were performed under galvanostatic conditions by applying 10 and 30 mA cm−2 using a MINIPA power supply under acidic conditions (0.5 mol L−1 H2SO4, for all experiments). PbO2 was used as anode (a plate with an area of 20 cm2), while a Ti grid was employed as cathode with an inter-electrode distance of 1.5 cm.
Results and discussion
Preliminary electrochemical measurements and surface analysis of PbO2 electrode
Important information on the electroactivity of anode surface material can be obtained prior to anodic oxygen evolution (o.e.) by polarization curve studies. As it can be observed from Fig. 2, potentiodynamic experiments indicated that Pb/PbO2 material presents higher oxygen overpotential, which implies that this anode is better electrocatalyst for H2Q oxidation. Analyzing the current densities values used in this work for anodic oxidation of H2Q oxidation, it can be inferred that at 10 mA cm−2, lower production of hydroxyl radicals could be attained. Conversely, higher values of applied current density favors higher production of hydroxyl radicals, consequently, good performances on electrochemical oxidation must be achieved. This assertion is in agreement with the results reported by other authors [23, 24]. Restricting now our analysis to the behavior of potential (see Fig. 2a), lower values of applied current densities promotes a good potential stability. Conversely, when 60 mA cm−2 was applied, the electrical potential was stable in the beginning of the electrolysis, after that, an important increase on potential was observed, indicating instability of the anode surface. However, this behavior will be discussed in next sections.
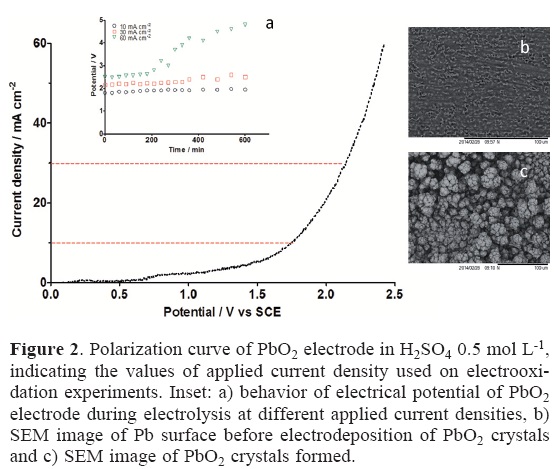
Regarding the anode surface obtained, Fig. 2b shows the SEM image of lead surface before the electrodeposition of PbO2 with a homogeneous surface without relevant alterations. On the other hand, the amount of PbO2 formed is evident by small PbO2 crystals observed by SEM image, showing the characteristic tetrahedral crystal structure (Fig. 2c).
Electroanalysis methodology: DPV measurements for H2Q
Before EO experiments, standardization and optimization of electroanalytical approach was performed in order to use the PbO2 sensor for the quantification of H2Q during its EO at Pb/PbO2 anode. To estimate electrochemical characteristics of PbO2 electrode and decide the working range of potentials, DPV curves were recorded in 0.5 mol L−1 H2SO4 and in solutions containing H2Q.
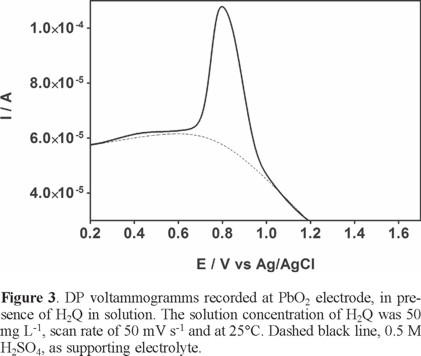
The working range of potentials was delimited from 0.0 to 1.2 V. Fig. 3 shows the voltammograms of 0.5 mol L−1 H2SO4, in the absence (dashed line) and presence of H2Q (bold line), recorded on PbO2 electrode. As can be seen, in the absence of H2Q, no peak was observed. However, in the presence of H2Q, an irreversible oxidation peak at ≈0.78 V (vs. Ag/AgCl) was observed on PbO2 electrode, attributed to EO of H2Q. These experiments showed that H2Q is electroactive at this material, its oxidation taking place about 1020-1100 mV before the oxygen evolution reaction (o.e.r.).
A linear relationship between peak current and H2Q concentration was obtained using PbO2 electrode (see Fig. 4). Calibration plot were recorded in a large concentration range to explore the dynamic and linear ranges: after the expected linear tendency at the lower concentration levels the slope diminished up to reach an asymptotic value. Each curve was obtained by evaluating the peak intensity as a function of the analyte concentration, and considering at least sixteen analyte concentrations (inset in Figure 4). The calibration plot was linear between 3.9±0.14 and 50±0.15 mg L−1, with regression coefficients always larger than 0.9981. The functional relationship was i/A=(9.93±0.11)×10−7 [H2Q] M + (1.56±0.33)×10−6 (slope and intercept were the average of six independent calibrations).
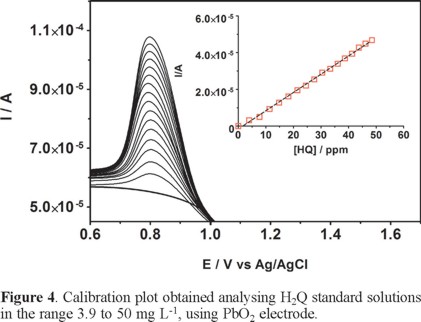
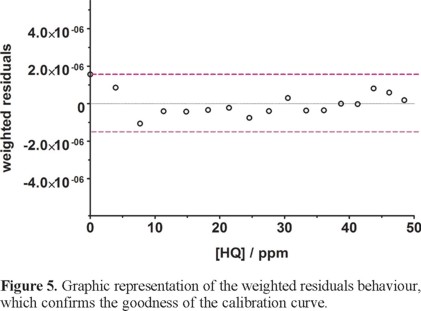
Figure 5 also shows that the residuals of the regression are randomly distributed around the zero, allowing a visual verification of the absence of a significant non linearity [25, 26]. It is worth noting that no significant differences in calibration curves recorded in different days were evidenced. A preliminary estimation of the Limits of Detection, LOD, was also possible by using the approach based on the standard deviation of regression [25, 26]: LOD = 3 × Sy/x / b, where Sy/x is the residual standard deviation and b is the slope of the calibration plot. On the basis of the results obtained, a LOD of about 2.05±0.12 mg L−1 could be estimated. This approach allows to control both false positive and false negative errors (a = b = 0.05) [25].
Electrochemical oxidation of H2Q
In order to study the anodic oxidation of H2Q, a set of galvanostatic electrolysis were performed at 10 and 30 mAcm−2 at 25 °C. During each electrolysis, samples of anolyte were withdrawn and analyzed by DPV analysis to quantify the concentration of H2Q remaining in the solution.
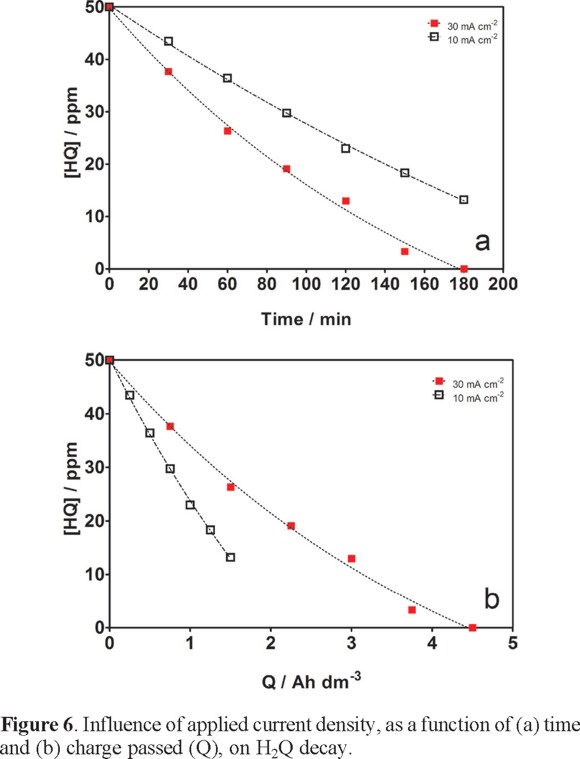
As can be seen from Figure 6a, at PbO2 anode, one of the extreme examples of classical high-oxygen-overpotential material and therefore expected to perform quite well in electrochemical mineralization of organics [6-8, 21, 24], 100% and 80% of mineralization of the initial H2Q amount in 180 min was attained by applying 30 and 10 mA cm−2, respectively. From the results reported in Figure 6b, the organic substrate is mineralized, at PbO2, upon consumption of 4.5 Ah dm−3 at 30 mA cm−2 compared with incomplete elimination attained at 10 mA cm−2 (∼ 2.5 Ah dm−3). This experimental evidence supports the idea that the surface of PbO2 favors the production of •OH radicals, after that, these strong oxidant species react with the organic substrate promoting a fast incineration [12, 24]. Nevertheless, under soft current conditions (10 mA cm−2), no enough production of hydroxyl radicals was attained, favoring the formation of hydrated lead dioxide surface, PbO(OH)2 [12, 21, 23, 24, 27, 28]. This assumption is in agreement with the potentiodynamic measurements showed in Fig. 2.
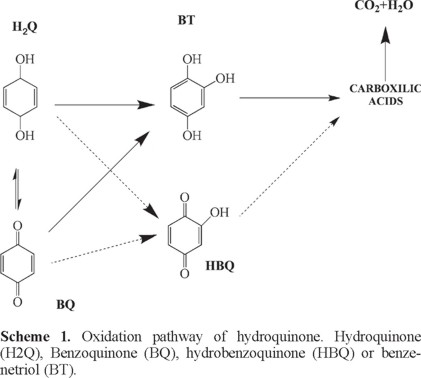
Based on the existing literature [6, 7, 23, 24], at PbO2 anode, no H2Q adsorption is attained, due to "non-active" nature of this electrode, but the organic oxidation clearly involves intermediates that are only available during the oxygen evolution reaction (hydroxyl radicals). Also, the degradation pathway reported by other authors [23, 29, 30] indicated that hydroquinone (H2Q) is rapidly oxidized to carboxylic acids (scheme). Only one intermediate is previously formed before complete aromatic ring fragmentation, however, this by-product is not enough stable for remain in solution, preferring its oxidation. This assertion is confirmed by no additional peaks observed at DPV analysis, indicating the no eventual formation of hydrobenzoquinone (HBQ) or benzenetriol (BT).
It is important to mention that, for this electrocatalytic material, PbO2-carboxylic acids interaction can be assumed, the carbonyl groups can exhibit a short-range interaction with surface Pb(IV) sites favoring the elimination of carboxylic acids formed during H2Q oxidation, as demonstrated by other authors during degradation of diethyl phthalate [21], p-nitrophenol [31], chloranilic acid [32], and glucose [33].
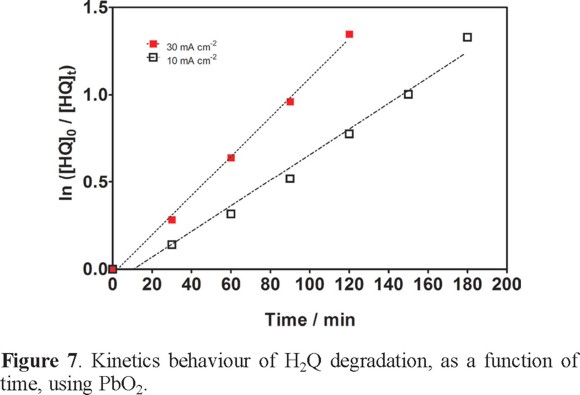
In order to strengthen this statement, the H2Q concentration decays were analyzed by kinetic equations related to simple reaction orders and good linear correlations were found by using a pseudo-first-order reaction, as presented in inset in the Fig. 7. As can be seen, the apparent rate constants of H2Q decay (kapp) increases when an increase on applied current density was attained. In fact, the apparent rate constant of 7.3×10−3 min−1 at 10 mA cm−2 passes to 1.1×10−2 min−1 at 30 mA cm−2, confirming the higher removal efficiency at higher current density.
As warmly suggested by other authors [25], the analytical procedures were applied by maintaining the analytical system under rigorous statistical control. Thus, the error related with the determination of H2Q, using DPV and HPLC procedures, was estimated as:
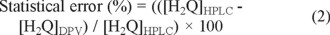
where [H2Q]DPV and [H2Q]HPLC are the concentrations of H2Q determined with HPLC and DPV, respectively.
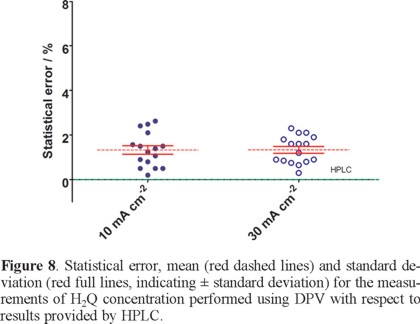
As it can be seen in Fig. 8, the DPV gave reliable results, the statistical error being below 5% in the majority, i.e. 98%, of the responses; in addition, it is important to highlight that the average value of H2Q concentration estimated by DPV, at all currents applied, lies with the concentration of H2Q expected (obtained by HPLC).
Conclusions
In conclusions, it was possible demonstrating the potentiality of the proposed electroanalytical procedure for determining H2Q during its EO. Such a sensor is characterized by a higher sensitivity and reproducibility and the low limit of detection allows reducing matrix effects by working in highly diluted solutions. Moreover, the proposed procedure is cheaper than the commonly used chromatographic analysis and than other instrumental methods involving more toxic or expensive reagents.
In order to compare the results obtained with the proposed procedures, HPLC experiments were performed to analyze the total amount of H2Q after electrolysis. Ten samples, during electrolysis at 10 and 30 mA cm−2, were collected and analyzed. According to the Student's t-test, there were no significant differences between the HPLC and electroanalytical procedures at a 95% confidence level.
Restricting now our analysis to EO of H2Q, the present results demonstrate that the process is strongly dependent on the applied current density. Participation of hydroxyl radicals, formed at PbO2 surface, is an important pre-requisite to an efficient mineralization of H2Q, demonstrating that this non-active anode is an ideal material for wastewater treatment.
Finally, the H2Q solutions after electrolysis, at 10 and 30 mA cm−2) were analyzed by electroanalytical methodology reported by Eiband and co-workers [34], and no evidences of pollution by Pb+2 were detected. Conversely, when a electrolysis test was performed at 60 mA cm−2, the contamination of final solution by Pb+2 was achieved. This assumption is confirmed by the electrochemical stability tests performed (see Fig. 2a) before the electrooxidation experiments for elimination of H2Q, where the electrical potential increases when higher values of applied current densities were used. These experiments also confirm the applicability of electrochemical oxidation treatment as an alternative for depollution of effluents using PbO2 anode under soft current conditions to avoid Pb+2 pollution.
Acknowledgements
A.J.S. and D.K.S.X. gratefully acknowledge the CAPES for Master and PhD Fellowships, respectively. The authors thank the financial support provided by PETROBRAS. Financial support from National Council for Scientific and Technological Development (CNPq-Brazil) is gratefully acknowledged. The authors are in debt to Dr. Artejose Revoredo da Silva for the analysis of surface morphology.
References
1. Xie, T.; Liu, Q.; Shi, Y.; Liu, Q. J. Chromatogr. A 2006, 1109, 317-321. [ Links ]
2. Wang, J.; Park, J.N.; Wei, X.Y.; Lee, C. W. Chem. Commun. 2003, 628-629. [ Links ]
3. Khachatryan, L.; Adounkpe, J.; Maskos, Z.; Dellinger, B. Environ. Sci. Technol. 2006, 40, 5071-5076. [ Links ]
4. Zhao, G.; Li, M.; Hu, Z.; Li, H.; Cao, T. J. Mol. Catal. A 2006, 255, 86-91. [ Links ]
5. Comninellis, C.; Guohua, C. (Eds.), Electrochemistry for the Environment; Springer: Berlin, 2009. [ Links ]
6. Panizza, M.; Cerisola, G. Chemical Reviews. 2009, 109, 6541-6569. [ Links ]
7. Martínez-Huitle, C.A.; Ferro, S. Chemical Society Reviews. 2006, 12, 1324-1340. [ Links ]
8. Chen, G. Separation and Purification Technology. 2004, 38, 11-41. [ Links ]
9. Martínez-Huitle, C. A.; Brillas, E. Angew. Chem. Int. Ed. 2008, 47, 1998-2005. [ Links ]
10. Brillas, E.; Martinez-Huitle, C. A. (Eds); Synthetic Diamond Films: Preparation, Electrochemistry, Characterization and Applications, Wiley, 2011. [ Links ]
11. Martinez-Huitle, C. A.; Brillas, E. Appl. Catal. B. 2009, 87, 105-145. [ Links ]
12. Martinez-Huitle, C. A.; Panizza, M. Application of PbO2 anodes for wastewater treatment, in Advances in Chemistry Research (Ed: D. V. Zinger), Vol. 1, Nova Science Publishers, Inc., New York 2010, 269-299. [ Links ]
13. Comninellis, C.; De Battisti, A. J. Chim. Phys. 1996, 93, 673-679. [ Links ]
14. Simond, O.; Schaller, V.; Comninellis, C. Electrochim. Acta. 1997, 42, 2009-2012. [ Links ]
15. Foti, G.; Gandini, D.; Comninellis, C.; Perret, A.; Haenni, W. Electrochem. Solid St. 1999, 2, 228-230. [ Links ]
16. Comninellis, C. Electrochim. Acta 1994, 39, 1857-1862. [ Links ]
17. Stucki, S.; Kotz, R.; Carcer, B.; Suter, W. J. Appl. Electrochem. 1991, 21, 99-104. [ Links ]
18. Quiroz, M. A.; Reyna, S.; Sanchez, J. L. S. J Solid State Electrochem. 2003, 7, 277-282. [ Links ]
19. Correa-Lozano, B.; Comninellis, C.; De Battisti, A. J. Appl. Electrochem. 1997, 27, 970-974. [ Links ]
20. Figueiredo, R. S.; PhD Thesis, Confecção e caracterização de eletrodos tridimensionais de PbO2 e PbO2/SnOx produzidos por anodização para decomposição de compostos orgânicos, Facultade de Engenharia Mecânica, UNICAMP, Brazil, 2014. [ Links ]
21. Vazquez-Gomez, L.; De Battisti, A.; Ferro, S.; Cerro, M.; Reyna, S.; Martínez-Huitle, C.A.; Quiroz, M.A.; Clean - Soil, Air, Water 2012, 40, 408-415. [ Links ]
22. Cerro-Lopez, M.; Meas-Vong, Y.; Méndez-Rojas, M.A.; Martínez-Huitle, C.A.; Quiroz, M.A.; Appl. Catal. B: Environ. 2014, 144, 174-181. [ Links ]
23. Nasr, B.; Abdellatif, G.; Cañizares, P.; Sáez, C.; Lobato, J.; Rodrigo, M.A.; Environ. Sci. Technol. 2005, 39, 7234-7239. [ Links ]
24. Panizza, M.; Cerisola, G.; Appl. Catal. B: Environ. 2007, 75, 95-101. [ Links ]
25. Miller, J. C.; Miller, J. N. Statistics for Analytical Chemistry, Vol. 3 (Ed: E. Horwood), PTR Prentice Hall, New York 1993. [ Links ]
26. Araújo, E.G.; Jhones dos Santos, A.; da Silva, D.R.; Salazar, R.; Martínez-Huitle, C.A.; Electroanalysis 2014, 26, 748-755. [ Links ]
27. Pavlov, D.; Monahov, B. J. Electrochem. Soc. 1996, 143, 3616-3629. [ Links ]
28. Pavlov, D. J. Electrochem. Soc. 1992, 139, 3075-3080. [ Links ]
29. Moctezuma, E.; Zermeño, B.; Zarazua, E.; Torres-Martínez L. M.; García, R.; Top. Catal. 2011, 54, 496-503. [ Links ]
30. Devillers, J.; Boule, P.; Vasseur, P.; Prevot, P.; Steiman, R.; Seigle-Murandi, F.; Benoit-Guyod, J.L.; Nendza, M.; Grioni, C.; Dive, D.; Chambon, P.; Ecotoxicol. Environ. Saf. 1990, 19, 327-354. [ Links ]
31. Quiroz, M. A.; Reyna, S.; Martinez-Huitle, C. A.; Ferro, S.; De Battisti, A.; Appl. Catal., Environ. B 2005, 59, 259-266. [ Links ]
32. Martinez-Huitle, C. A.; Quiroz, M. A.; Comninellis, C.; Ferro, S.; De Battisti, A.; Electrochim. Acta 2004, 50, 949-956. [ Links ]
33. Bonfatti, F.; Ferro, S.; Lavezzo, F.; Malacarne, M.; Lodi, G.; De Battisti, A.; J. Electrochem. Soc. 1999, 146, 2175-2179. [ Links ]
34. Eiband, M.M.S.G.; De A. Trindade, K.C.; Gama, K.; Melo, J.V.D.; Martínez-Huitle, C.A.; Ferro, S.; J. Electroanal. Chem. 2014, 717-718, 213-218. [ Links ]














