Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.58 no.3 Ciudad de México jul./sep. 2014
Article
Compost Aided Electrokinetic Remediation of an Hydrocarbon Polluted Soil
Ivonne Duarte Medina,1 Erika Bustos Bustos,2 and Margarita Teutli León1,*
1 Facultad de Ingeniería, Benemérita Universidad Autónoma de Puebla, Blvd. Valsequillo esq. Av. San Claudio, Puebla 72570, México. teutli23@hotmail.com
2 Laboratorio de Tratamiento de Suelo, Centro de Investigación y Desarrollo Tecnológico en Electroquímica, S. C. Parque Tecnológico Querétaro, Sanfandila, Pedro de Escobedo, Querétaro, México.
Received January 14th, 2014
Accepted April 23rd, 2014.
Abstract
An electrokinetic treatment was applied to a weathered hydrocarbon polluted soil compost amended. Results have shown an enhancement in hydrocarbon removal since initial concentration was 18700 mg Kg−1, electroremediated soil ended with 7410 mg-Kg−1, while the compost aided electroremediated soil lowered its concentration to 3250 mg-Kg−1. GC-MS soil analysis evidenced complex molecules at the anode section, while simplest molecules were at the cathode section, in this section survival of Eisenia Andrei worms was higher than 90%.
Key words: electrokinetics, compost, hydrocarbon polluted soil, electrolyte, residual toxicity.
Resumen
Se aplicó tratamiento electrocinético a suelo intemperizado contaminado con hidrocarburos y mezclado con composta. Los resultados muestran que la remoción de hidrocarburos se favoreció yendo de 18700 mg Kg−1 iniciales a 7410 mg-Kg−1 al electrorremediar, inclusión de composta permitió bajar a 3250 mg-Kg−1. Análisis de GC-MS evidenciaron que las moléculas complejas están en la sección anódica, mientras que las moléculas simples están en el cátodo, y en esta sección la sobrevivencia de la lombriz Eisenia Andrei fue mayor a 90%.
Palabras clave: electrocinética, composta, suelo contaminado con hidrocarburos, electrolito, toxicidad residual.
Introduction
Petrochemical industry expansion has been a source for high amounts of complex, toxic compounds, such as the polycyclic aromatic hydrocarbons (PAHs). In places where oil spills have taken place it has been found that these environmental pollutants stand sorbed in the soil matrix, being at such higher concentrations that a toxic environment is created and microbial populations no longer stand.
At southeast Mexico, soils have been subject of strong, negative impacts due to spills having place during oil exploration and exploitation, being a priority to search for remedial options. Actually, for soil remediation are technologies focused on isolation/ destruction of pollutants throughout chemical structure modifications by applying either thermal, biological or chemical treatments, but most of the times these techniques require long time periods to be applied.
Electrokinetic soil remediation allows to remove organic pollutants like hydrocarbons by placing a pair of electrodes insight the soil matrix, such that by closing the electrical circuit an electric field can be generated forcing ion movement to the opposite charge electrode (electromigration) water movement in respect to soil particles (electroosmosis) as well as charged soil particles movement (electrophoresis) [1, 2].
A concern about electrokinetics is that in the case of clays the electric field can induce changes on the soil matrix structure leading to a reduction in electroosmotic flow, as consequence of the interactions at the interphase soil-electrolyte [3, 4].
Another research goal has been to assure that electrokinetics does not leave an sterile soil, this means that some microbial species should be able to grown up in treated soil, a report that provides evidence about how electrokinetics enable microbial population is the one of Oszust [5], where the authors report that total number of bacteria and fungi has increased in respect to the untreated soil.
Because polluted soils disposal can result in an environmental problem and health risk for humans, researchers are looking for soil remediation techniques in which not only pollutants could be removed but also remediated soil could recover some of its unpolluted properties. In this sense, bioremediation is an useful tool that can be applied on the basis of using local microorganisms, which in some sense have acquired some kind of resistance to the specific pollutant in the site. There is a report focused on hydrocarbon polluted soil bioremediation based on mixing polluted soil with compost [6], authors have pointed out that this mixture allows accelerating hydrocarbon biodegradation if soil physicochemical conditions are optimized by controlling: (a) nutrient addition represented by organic matter content, (b) pH, and (c) temperature.
Successful soil bioremediation for hydrocarbon polluted soils can be attained if a surfactant is included either in the electrolyte or into the soil matrix. An example of surfactant in the electrolyte has been reported [7], in which the author treated an hydrocarbon polluted soil by three methods and set up a comparison between electroremediation, soil washing with Triton -X-114 surfactant and bioremediation. From these results it is concluded that a higher removal can be obtained with electrokinetics but it is the most expensive treatment leaving the soil with a moderate toxicity; otherwise, soil washing provides the lower removal, but it leaves a soil with higher toxicity; finally bioremediation provides an intermediate removal, but it is the one that leaves lower toxicity.
Also, there are reports about that humic and fulvic acids can be considered natural surfactants [8, 9], and they can be used in washing of highly contaminated soils [10].
In this paper is reported a study of hydrocarbon removal from a weathered polluted soil collected at an industrial site located at the south of Veracruz, Mexico. Experimental approach considered to perform a batch screen by using 5 wetting solutions, 4 reaction times, and 5 dosages of compost; from these results, it was chosen the best combination of conditions for testing hydrocarbon electrokinetic removal efficiency, considering two conditions: natural soil and compost amended soil. It is expected that compost organic matter will act like a natural surfactant allowing an increase in the amount of desorbed hydrocarbons from the soil matrix.
Results and discussion
As a first step a soil physical characterization was done, experimental procedures and classification are based on the NOM-021 SEMARNAT 2000 [11], this allowed to know granular composition of the soil matrix structure. Otherwise, the amount of hydrocarbons was quantified like grease and oil (G&O) by the Soxhlet method, according to the methodology in the Mexican norm NMX-AA-134-SCFI-2006 [12], results are reported in Table 1.

Determination of the optimal soil:compost ratio
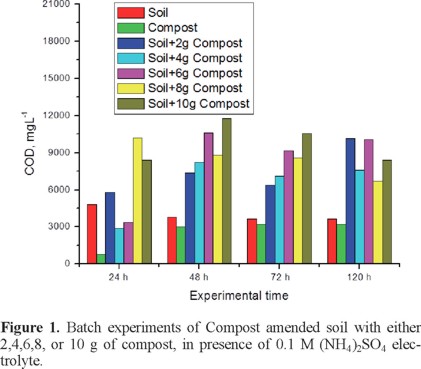
This was estimated by running a set of batch experiments using (NH4)2SO4 0.1 M as electrolyte, two blanks one of 20 g of soil (S), and one of 20 g of compost (C), and combinations of 10 g soil plus X g of compost, being X=2,4,6,8,10 g; in these experiments it was observed gas evolution during the first 48 h, permeated solution was collected and analyzed for Chemical Oxygen Demand (COD) parameter, as a first approach for estimating pollutant solubilization, results are shown in Figure 1; from the plot it can be observed that maximum pollutant solubilization was obtained at 48 h, and the optimal ratio soil:compost was 10:6, which corresponds to 34% of compost in amended samples.
Determination of optimal electrolyte
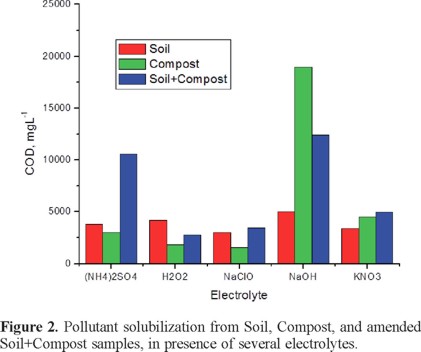
Once the soil:compost ratio was established, the next step was to evaluate the best electrolyte. Selection was done by running a set of batch experiments with 0.1 M solutions of either Na2SO4, H2O2, HClO, NaOH or KNO3, results are shown in Figure 2. For this set of experiments it was considered one blank for soil and one for compost, as well as the amended soil with compost at the optimal ratio 10:6 (34% weight); as it can be observed, NaOH is the best electrolyte since it provides the higher solubilization for the three samples Soil, Compost, and Soil+Compost. The next option in electrolyte would be the (NH4)2SO4, since it provides a high hydrocarbon solubilization in the amended Soil+Compost sample; although, a very low amount of hydrocarbon solubilization was observed in either Soil or Compost.
Having chosen the soil:compost ratio and the electrolyte, it was run a set of potentiostatic experiments considering 6, 12, 18, 24, 27 and 30 Volts, correlating the obtained response in current, pH at the wells, and residual pollutant profile throughout the cell; from these results it was chosen to work at 6 volts, because this potential guarantees: a) low current which will act avoiding soil desnaturalization; b) low electroosmotic flow which provides longer residence time, therefore higher interaction soil-electrolyte; and c) an adequate concentration gradient between electrodes since pH drops to 2 at the anode and reached 13 at the cathode.
Soil electroremediation
The next step in this study was applying electrokinetics to both the natural and amended soils, soil was wetted with NaOH electrolyte 1 hour before running the electroremediation, a pair of IrO2-Ta2O5|Ti electrodes was used. In order to discriminate the amount of solubilized hydrocarbons from total solubilized pollutants, it was used the Grease and Oil (G&O) parameter determined by the Soxhlet technique. After electrokinetics soil sample was cut in three sections named anode, center and cathode, in reference at its position in respect to the electrodes. Each section was carefully homogenized, dried, weighted and placed in the Soxhlet system for G&O determination.
In Figure 3 are shown the hydrocarbon residual concentrations (G&O) in the soil from electrokinetic experiments run at 1, 2, and 3 h. It is included the wetted amended soil as reference. As it can be observed for 1 h experiments the residual concentrations, in the three sections, are about 6 g-Kg−1 which is the maximum obtained removal in respect to the 2 and 3 h experiments. In the 2 h experiment it is observed that residual hydrocarbons are higher than those in the 1 h experiment, but higher concentrations belong to the anode section. In the 3 h experiment it is evident that a flow polarization is taking place since residual concentrations at the anodic and cathodic sections are higher (17 and 14 g-Kg−1 respectively) than the one at the center section (8 g-Kg−1).

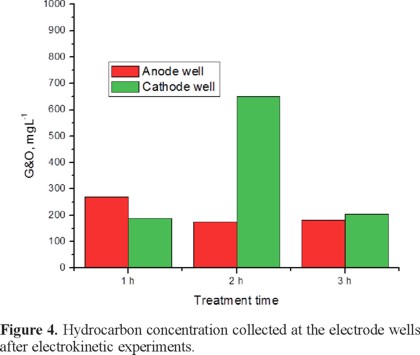
Considering that solubilized pollutants, according to its coordinated valence, can migrate either to anode or cathode electrodes, and coming accumulated at the electrode wells; then for each electrokinetic experiment, the solution from well electrodes was collected and evaluated for G&O. Results are shown in Figure 4. As it can be seen for the 1 hour experiment hydrocarbons collected at the anodic well are 40% higher than those collected at the cathodic well, then it can be inferred that solubilized pollutants exhibit negative charge being able to migrate in anodic direction. In opposite way the higher amount of hydrocarbons was collected with the 2 h experiment, in this transportation is taking place in cathodic direction since concentration was higher in the cathodic well, being almost three times the amount collected at the anodic well. Finally the amounts collected at the electrode wells from the 3 h experiment are the smaller ones, and very close one to another, fact that confirm that effectively at 3 h of treatment a bidirectional flow can be induced.
Modifications in well electrolyte and wetting time
In order to evaluate if hydrocarbon removal efficiency can be enhanced by changing the electrolyte at the wells, or by allowing the wetted sample stay longer before applying electrokinetics, some experiments were run considering water instead of NaOH at the wells, and soil wetting 24 h before applying electrokinetics.
Results are shown in Figure 5, it was observed that the higher removal was obtained with amended soil wetted 1 h before applying electrokinetics, and having fill electrode wells with NaOH electrolyte (1h+C+NaOH well); also it becomes evident that compost absence (1h+NaOH well) lowered removals at almost 50% in respect to the amended soil; additionally it was observed that even though the pH at the wells is above 12; after 1 h soil pH nearby the anode drops to 2, while at the center section pH is about 8, and in the cathodic zone pH is above 12; considering that these pH values will be aggressive to living organisms, it was contemplated to wet the soil with NaOH, but placing water at the electrode wells, and let stand for 24 hours before applying electrokinetis (24h+C+H2O well; 24h+H2Owell), with this option it is possible to get less aggressive pH values; since a pH of 11 was registered at the cathodic zone, and pH at the anodic zone drops only to 3, additionally this option could allow to eliminate the compost since removals are similar for both experiments (with/without compost), and these are about 80% of the recoveries obtained with the optimal process represented by compost amended soil plus NaOH electrolyte at the electrode wells

From these results, it was considered that the best option to expose living organisms is the one of amended soil wetted with NaOH, let stand 24 hours, and use water to fill electrode wells.
Organic compounds determination
In order to determine the organic compounds present in the soil before and after the electrokinetic treatment, all recovered G&O samples from each section were analyzed by gas chromatography coupled to mass spectrometry (GS-MS) according to the procedure described in the Mexican norm NOM-138-SEMARNAT/SSA1/2012 [13]. Results are presented in Figure 6, as it can be observed hydrocarbon compound concentrations are subject of variations in concentration, such that the greater compound acumulation is occurring in the anodic section, and the most abundant compound is the triacontane which is a high molecular weight alkane, appearing during gasoline production; also, it can be observed that the middle zone corresponding to the named center section, it is the one having the higher number of products, most of them being short aliphatic hydrocarbon chains like propane, ethane; finally in the cathodic region there are few methyl molecules being the main one the hidroxymethyl.

Evaluation of toxicity in electroremediated soil
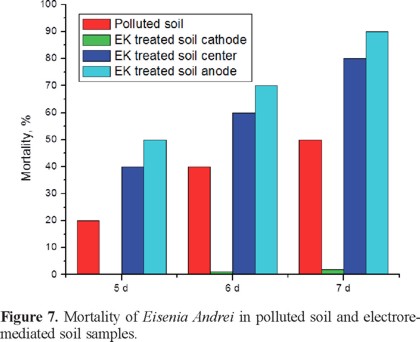
A presuntive toxicity test using Eisenia Andrei worms was run with electroremediated soil. In this test it was used worms older than 2 months, which were exposed to the polluted and electroremediated soil from the three sections anode, center and cathode. Results are shown in Figure 7, as it can be observed worms in the polluted soil were dying progressively registered percentages were 20% at the 5th day, 40% at the 6th day and 50% at the 7th day. Also the higher mortality was observed at the anodic sample which is the section having higher molecular weight compounds; at the center section mortality was lower than the one observed at the anodic section, but higher than the one in untreated soil. Also a survival greater than 90% was observed in the cathodic sample, this values agreed with observations from the previously discussed data, since this sample contains simpler molecules most of them of 1 carbon.
Conclusion
Experimental data obtained provided evidence that by using compost to amend a weathered hydrocarbon polluted soil, it is possible to enhance hydrocarbon solubilization and removal, the best response was obtained with a 34% weight of compost. The best electrolyte for wetting soil samples was NaOH, with this electrolyte both natural and compost amended soils exhibit the higher compound solubilization, estimated with COD test, as an alternative electrolyte it can be recommended the (NH4)2SO4 which exhibit a synergy when the sample is compost amended. Experimental recommended time in electrokinetic experiments is 1 h for obtaining the higher hydrocarbon removal, as original concentration of 18.7 g-Kg−1, was lowered to 7.41 g-Kg−1 in electroremediated natural soil, and to 3.25 g-Kg−1 in compost amended soil; but also 2 h of electrokinetic treatment can be useful to collect higher amounts of hydrocarbons in the electrode wells.
Use of GC-MS was useful to determine the relative abundance and type of hydrocarbons at each soil section after being electroremediated, from these results it was observed that the higher amount of high molecular weight compounds are present in the anodic section which resulted of high toxicity to Eisenia Andrei worms; at the central section main compounds are alkanes exerting medium toxicity onto worms; finally the cathodic section with low concentration of methyl compounds was the one where worms survival was greater than 90%.
Acknowledgements
Ivonne Duarte thankful by being assigned a scholarship sponsored by CONACyT and Veracruz Government through the project named: "Electrorremediación de suelo contaminado con hidrocarburos en las instalaciones de la industria petroquímica en Coatzacoalcos, Veracruz".
References
1. Pazos, M.; Sanromán, M.; Cameselle, C. Chemosphere 2006, 62, 817-822. [ Links ]
2. Yeung, A.; Bricka, M. J. Environ. Eng. ASCE, 1999, 125, 27-35. [ Links ]
3. Loch, J. P.; Lima, A. T.; Kleingeld, P. J. J. Appl. Electrochem, 2010, 40, 1249-1254. [ Links ]
4. Sumbarda Ramos, E. G.; Guerrero-Gutiérrez, O. X.; Murillo-Rivera, B.; González, I.; Oropeza-Guzmán, M. T. J. Appl. Electrochem, 2010, 40, 6, 1255-1261. [ Links ]
5. Oszust, K.; Frac, M.; Ochoa, B.; Cárdenas, J.; Teutli, M.; Bustos, E. in: Recent Research Developments in Electrochemistry, Vol 9, Pandalai, S. G. Ed. Transworld Research Network, 2013, 33-48. [ Links ]
6. Velasco, J.; Volke, T. Biodegradación de hidrocarburos de petróleo en suelos intemperizados. SEMARNAT, CENICA, INE, 2003. [ Links ]
7. Alba, G. I.; Cuevas, M. C.; Bustos, E. Int. J. Electrochem. Sci., 2013, 8, 4735-4746. [ Links ]
8. Quadri, G.; Chen, X.; Jawitz, J. W.; Tambone, F.; Genevini, P.; Faoro, F.; Adani, F. Environ. Sci. Technol., 2008, 42, 2618-2623. [ Links ]
9. Campitelli, P. A.; Velasco, M. I.; Ceppi, S. B. Talanta, 2006, 69, 1234-1239. [ Links ]
10. Conte, P.; Agretto, A.; Spaccini, R.; Piccolo, A. Environ. Pollut., 2005, 135, 515-522. [ Links ]
11. NOM-021-SEMARNAT-2000. Especificaciones de fertilidad, salinidad y clasificación de suelos. Estudios, muestreo y análisis. [ Links ]
12. NMX-AA-134-SCFI-2006 Suelos-hidrocarburos fracción pesada por extracción y gravimetría-método de prueba. [ Links ]
13. NOM-138-SEMARNAT/SSA1-2012. Límites máximos permisibles de hidrocarburos en suelos y lineamientos para el muestreo en la caracterización y especificaciones para la remediación. [ Links ]














