Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.58 n.3 Ciudad de México Jul./Sep. 2014
Article
Electrochemical Oxidation of 4-Chlorophenol Over a Carbon Paste Electrode Modified with ZnAl Layered Double Hydroxides
Daniel Hernández-Fuerte,1 Manuel Palomar-Pardavé,1,* Teresa de Jesús Licona-Sánchez,1 Mario Romero-Romo,1 and Jaime S. Valente2
1 Universidad Autónoma Metropolitana-Azcapotzalco, Departamento de Materiales, Av. San Pablo 180 Col. Reynosa-Tamaulipas, C. P. 02200, México D. F. México. mepp@correo.azc.uam.mx.
2 Instituto Mexicano del Petróleo, Eje Central # 152, 07730 México D. F., México.
Received January 31st, 2014
Accepted April 4th, 2014.
Abstract
A study is presented on the electrochemical oxidation of 4-chlorophenol (4cp) in aqueous solution using a bare carbon paste electrode, CPE, and another one that was modified with ZnAl layered double hydroxides (CPE/ZnAl-LDH). The electro-oxidation was effected at pH values ranging from 3 up to 11. It was found through cyclic voltammetry that this process was irreversible, namely, there were no reduction peaks, and that depending on the nature of the electrode, the anodic current was limited either by adsorption (CPE) or diffusion (CPE/ZnAl-LDH). The energy required and the oxidation reaction rate depended on the pH and on the nature of the electrode, such that the greater rates were obtained when the CPE/ZnAl-LDH electrode and acid pHs were used.
Key words: 4-chlorophenol; cyclic voltammetry; carbon paste electrode; ZnAl layered double hydroxides, pH.
Resumen
Se presenta un estudio sobre la oxidación electroquímica de 4-clorofenol (4cp) en disolución acuosa utilizando dos tipos de electrodos: uno de pasta de carbono simple, EPC; y otro modificado con hidróxidos dobles laminares de ZnAl (EPC/ZnAl-LDH). La electro-oxidación se llevó a cabo a valores de pH en el intervalo de 3 a 11. Se encontró a través de voltamperometría cíclica que este proceso es irreversible, es decir no se detectó la presencia de picos de reducción, y que dependiendo del tipo de electrodo el proceso anódica está limitada por la adsorción (EPC) o por la difusión (EPC/ZnAl-LDH). La energía requerida y la velocidad de la reacción de oxidación dependen tanto del pH como del tipo de electrodo de tal manera que las mayores velocidades fueron medidas cuando se utilizó el electrodo EPC/ZnAl-LDH en medios ácidos.
Palabras clave: 4-clorofenol; voltamperometría cíclica; electrodo de pasta de carbono; hidróxidos dobles laminares de ZnAl, pH.
Introduction
Presently the undergoing pollution in residual waters has become quite important to the extent that several different methods have been proposed to clean up the effluents from various organic and inorganic pollutants. Recently, the former pollutants have become of particular interest, e.g. the phenols and their derivative, such as the 4-chlorophenol, 4cp. 4cp is used for fabricating insecticides and for preserving wood, although the largest quantities of 4cp are as a byproduct from the paper pulp bleaching process, mainly because of the inherent properties associated to the chlorine presence [1,2]. In view of the persistence of 4cp throughout the environment and due to its low biodegradability, it is required to devise an effective, inexpensive method, easy to apply for its degradation.
Several different degradation methods have been proposed for 4cp [3], mainly the photocatalysis with TiO2, that of advanced oxidation using ozone and oxidation through Fenton reaction, among others [4]. In spite of the notable advantages that these methods display, only a few electrochemical processes have been proposed to effect the electrochemical degradation of phenols [1,5,6]. Among these it is worth to underline those which use granular graphite as electrode (oxidation of 4cp) [1], hydrotalcites-modified (phenol oxidation) [5] and unmodified glassy carbon (oxidation of 4cp) [6]. Considering the advantages displayed by the semiconducting materials to promote the oxidation of organic compounds, the ZnAl layered double hydroxides, ZnAl-LDH, have been recently reported as an excellent alternative for oxidation of organic pollutants, with excellent results during the photocatalysis of the degradation of organics componds [7, 7bis, 8] and those associated to electrochemical methods, this work presents the results of the study on the electrochemical oxidation of 4cp using an unmodified carbon paste electrode, CPE, and a modified CPE containing ZnAl-LDH, CPE/ZnAl-LDH.
Experimental
Synthesis of ZnAl-LDH
This material was synthesized by means of co-precipitation at low oversaturation from basic K2CO3 (Fermont 99.0 %) and KOH (Fermont 85.0 %) 2 mol L−1 solutions and separately, a Zn(NO3)2·6H2O (Aldrich, 98.0%), Al(NO3)3 ·9H2O (Fermont 99.1%) solution, both of which were poured into a padded reactor with agitation and uniform heating, keeping a constant pH. The resulting precipitate was aged for one night. Subsequently it is filtered and washed with abundant bidistilled water and left to dry at 100 °C overnight [9, 10].
Instrumentation
A typical three-electrode electrochemical cell was used, with either CPE or CPE/ZnAl-LDH as working electrode. The manufacture of the actual base of the working electrode followed the method published elsewhere by Ramírez-Silva et al. [11,12]; a Pt wire served as counter electrode, while the usual Ag/AgCl/KCl(aq,sadt) served as reference electrode. The electrochemical oxidation of 4cp was analyzed out by means of cyclic voltammetry, CV, with KCl (Mallinckrodt 99.7%) as supporting electrolyte and the Potentiostat/Gavanostat PGSTAT 100 AUTOLAB.
Influence of the pH
The pH of the 4cp (Aldrich 99%) solution was set using HCl (Aldrich 36.5-38.0%) and/or NaOH (J.T. Baker 98.4%) such that it was brought to the following values: 3, 5, 7, 9 and 11, by means of using a sevenMulti Mettler Toledo AG potentiometer. All the experiments were performed at ambient temperature.
Characterization
The ZnAl-LDH was characterized by means of XRD aided by a Siemens D-500 diffractometer with Cu Kα radiation, operating at 35 kV and 25 mA. The sample was analyzed within the 4 to 80° angular position 2ϑ range, with a step width of 0.02° and counting time of 1 s/point.
The chemical composition of the samples was determined by means of a Perkin-Elmer instrument Optima 3200 Dual Vision inductively coupled plasma atomic emission spectrometry, ICP-AES.
Results and discussion
Characterization of ZnAl-LDH
Figure 1 shows the XRD diffractogram of the synthesized ZnAl-LDH. It can be observed the presence of the layered double hydroxide phase displaying good crystallinity. Also, the characteristic diffraction peaks of a LDH phase with the indexes (003) and (006) were identified, at low 2ϑ angle while the non-basal peaks were associated with the indexes (101), (015), (018), (110) and (113) at higher 2ϑ angles.

The chemical composition of the sample is given by the following expression [Zn0.72 Al0.28 (OH)2] (CO3)0.141.0 H2O, which gives a real value for the molar Zn2+/Al3+ ratio of 2.57, which was determined by plasma emission spectrometry.
Electrochemical study
Figure 2 shows two cyclic voltammograms obtained during the 4cp oxidation using the unmodified and the ZnAl-LDH-modified CPEs at the same pH value and scan rate. In both cases the potential scan started at 200 mV scanning in the positive direction. Note that 4cp oxidation is chemically irreversible, since no faradaic current was observed upon inverting the potential scan in the negative direction within the whole potential range explored. However, the anodic peak current obtained with the CPE/ ZnAl-LDH electrode was approximately 7 times larger than that corresponding to the unmodified CPE.

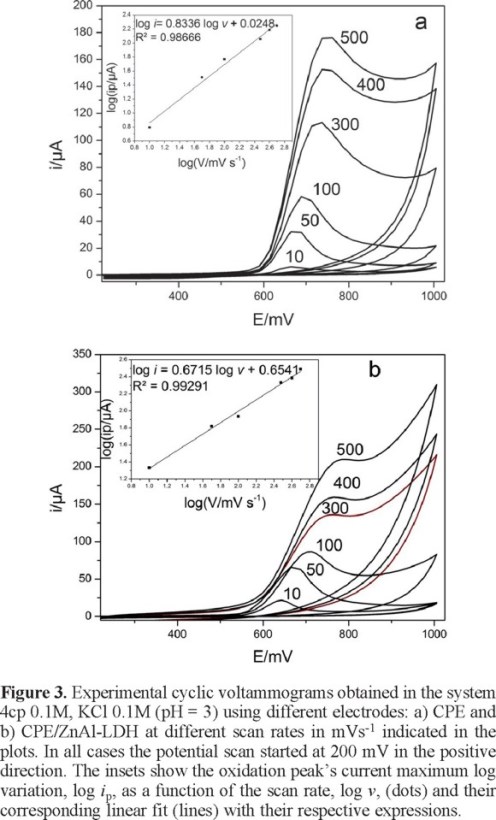
Influence of the scan rate
Figure 3 shows the effect of the scan rate on the features displayed by the cyclic voltammograms obtained during the 4cp oxidation using different electrodes. In the case of the unmodified CPE electrode, the variation of the log of the oxidation's peak current maximum, namely log ip, as a function of the log of the scan rate, log v, was linear with a 0.83 slope, see the inset in Figure 3a, this value indicates that the 4cp oxidation process taking place on this electrode would be controlled by the adsorption of 4cp (for a pure adsorption process a slope of 1 must be expected). However, in the case of the ZnAl-LDH-CPE modified electrode, the said slope has a value of 0.67, see inset in Figure 3b, which indicates that the oxidation process would be limited by mass transfer of the electroactive species toward the electrode's surface, namely their diffusion [13] (a pure diffusion process is associated with a slope value of 0.5).
Influence of the pH
Figure 4 shows the variation for both the ip and the 4cp anodic peak potential, Ep, after imposing different pH values in the working solution. Regardless of the electrode used, the Ep decreased with increasing pH thus decreasing the energy required to promote the process. As can be noted from scheme 1a, this clearly support that oxidation of the deprotonated form of 4cp is energetically easier that the protonated one. Similarly, the ip variation has a clear dependence with the nature of the electrode: for acid and neutral pH, the greatest 4cp oxidation rate was obtained with the CPE/ZnAl-LDH and a maximum at pH 5, although for basic pH values the greatest oxidation rate corresponded to the unmodified CPE giving a maximum that coincides practically with the pKa (9.2) [14] reported for the 4cp.


CP electrochemical mechanism
As we have shown in section 3.2.2 the solution pH drastically influences the electrochemical oxidation behavior of 4cp, which could be directly related with the predominant form of 4cp in solution, see a) in scheme 1. In the case where the bare CPE was used, at pH 3, see Figure 3a, the 4cp oxidation is controlled by adsorption of its neutral form. For systems where strong reactant adsorption is involved, the full peak width at half-height (EFWHM) should be equal to 90.6 mV/ n, thus from this value we estimate that the number of electrons involved in this case, n, was 1. Considering this experimental evidence we propose that in this case the 4cp electrochemical oxidation occurs through the loss of one electron to form the phenoxy radical, see b) in scheme 1. It is important to mention that Rodrigo et al., [15] have proposed that 4cp oxidation onto a boron-doped diamond electrode involves two-step one-electron oxidation of 4-CP to phenoxy radical and phenoxy cation. Notwithstanding, some questions regarding this mechanism are still open, namely: what could be the structure of the first species formed by electron transfer? And what could be the coupled reaction that confers the chemically irreversible character to the oxidation waves shown in figure 3? These very important aspects are at this moment the subject of investigation by our group.
Conclusions
It is possible to electrochemically oxidize the 4cp dissolved in aqueous solution using Zn-Al hydrotalcite-modified or unmodified carbon paste electrodes. Depending on the nature of the electrode used the oxidation process rate limiting step changes from being adsorption-limited (CPE) to being diffusion-limited when using the CPE/ZnAl-LDH-modified electrode. Further, the work carried out shows that regardless of the electrode used, the pH variation from acid to basic, induced decrements of the energy required to oxidize the 4cp, however, the oxidation rate was strongly dependent on the nature of the electrode and of the dissolution's pH. For acid and neutral pHs, the greatest rates were obtained with the CPE/ZnAl-LDH electrode, whereas for basic pHs these were obtained with the unmodified CPE. This study clearly shows that depending of the solution pH and the electrode used, the electrochemical oxidation of 4cp occurs with different rate (current) and different applied potential (energy), these parameter are quite important to take into consideration for the development of a robust electrochemical treatment that may be used for the remediation of wastewater containing 4cp.
Acknowledgements
DHF and TJLS thank CONACYT for their studentships to pursue their postgraduate and postdoctoral research work, respectively. MPP, MRR and JSV thank the SNI for the distinction of their membership and the stipend received. The authors thank the financial support received from the Departamento de Materiales, and special thanks are given to the Área Ingeniería de Materiales to carry out this project in its LIEIM laboratory.
References
1. Chen, J. L.; Chiou, G.C.; Wu, C. C. Desalination 2010, 264, 1-2, 92-96. [ Links ]
2. García-Molina, V.; Kallas, J.; Esplugas, S. Chem. Eng. J. 2007, 126, 59-65. [ Links ]
3. Herrmann, J. M.; Disdier J.; Guillard, C.; Laine, J.; Malato, S.; Blanco, J. Catal. Today 1999, 54, 255-265. [ Links ]
4. Valente, J. S.; Tzompantzi, F.; Prince, J. Appl. Catal. B: Environ. 2011, 102, 276-285. [ Links ]
5. Fernández, L.; Borrás, C.; Carrero, H. Electrochim. Acta 2006, 52, 3, 872-884. [ Links ]
6. Duan, X.; Tian, L.; Liu, W.; Chang, L. Electrochim. Acta, 2013, 94, 192-197. [ Links ]
7. Seftel, E. M.; Popovici, E.; Mertens, M.; Witte, K. D.; Tendeloo, G. V.; Cool, P.; Vansant, E. F. Materials. 2008, 113, (1-3), 296304. [ Links ]
7bis. Valente, J. S.; Tzompantzi, F.; Prince, J.; Cortez, J.G.H.; Gómez, R. Appl. Catal. B Environ. 2009, 90, 330-338. [ Links ]
8. Hadj Salah, N.; Bouhelassaa, M.; Bekkouche, S.; Boultii, A. Desalination 2004, 166, 347-354. [ Links ]
9. Cavani, F. Trifiro, F.; Vaccari, A. Catal. Today 1991, 11, 173-301. [ Links ]
10. Rives, V. Layered Double Hydroxides: Present and Future, Ed. Nova, Science Publishers 2001. [ Links ]
11. Ramirez, M.T.; Palomar. M. E.; Gonzáles I.; Rojas-Hernández A. Electroanal. 1995, 7, 184-188. [ Links ]
12. Martínez, R.; Ramírez, M.T.; González, I. Electroanal. 1998, 10, 336-342. [ Links ]
13. Gosser D.K. Jr., Cyclic Voltammetry Simulation and Analysis of Reaction Mechanisms VCH, Ed. Weinheim 1993. [ Links ]
14. Solomons, T. W. G. Química Orgánica, Ed. Limusa Wiley México, 2000. [ Links ]
15. Rodrigo, M. A.; Michaud, P. A.; Duo, I.; Panizza, M.; Cerisola, G.; Comninellis, Ch. J. Electrochem. Soc. 2001, 148, D60-D64. [ Links ]














