Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Journal of the Mexican Chemical Society
versão impressa ISSN 1870-249X
J. Mex. Chem. Soc vol.58 no.3 Ciudad de México Jul./Set. 2014
Article
Electrooxidation of Diclofenac in Synthetic Pharmaceutical Wastewater Using an Electrochemical Reactor Equipped with a Boron Doped Diamond Electrode
Gabriela Coria, José L. Nava,* and Gilberto Carreño
Universidad de Guanajuato. Departamento de Ingeniería Geomática e Hidráulica. Av Juárez 77, Zona Centro, 36000, Guanajuato, Guanajuato, México. jlnm@ugto.mx
Received February 1st, 2014
Accepted March 25th, 2014.
Abstract
This paper deals with the degradation of diclofenac by electrochemical oxidation in NaClO4 medium at neutral pH using a FM01-LC reactor equipped with a boron doped diamond electrode (BDD). Microelectrolysis studies were carried out to find the current density domain where hydroxyl radical (•OH) formation is favored, 10 ≤ j ≤ 20 mA cm−2. Electrolysis experiments at mean linear flow velocities of 14.6 ≤ u ≤ 58.4 cm s−1 were performed. The experimental set-up achieved 100% diclofenac mineralization with 78% current efficiency and energy consumption of 2.54 kWh m−3 at j = 15 mA cm−2 and u=29.2 cm s−1.
Key-words: Degradation of diclofenac, boron doped diamond electrode, water treatment, electrooxidation, FM01-LC reactor.
Resumen
Este trabajo muestra la degradación de diclofenaco por oxidación electroquímica en medio de NaClO4 y pH neutro empleando el reactor el FM01-LC equipado con un ánodo de diamante dopado con boro (BDD). Se llevaron a cabo estudios de microelectrólisis para determinar el dominio de densidad de corriente donde se favorece la formación de radicales hidroxilo (•OH), 10 ≤ j ≤ 20 mA cm−2. Se realizaron electrolisis a velocidades promedio de flujo comprendidas entre 14.6 ≤ u ≤ 58.4 cm s−1. La degradación completa de diclofenaco (100%) se logró a 15 mA cm−2 and u=29.2 cm s−1, con una eficiencia de corriente de 78% y un consumo de energía de 2.54 kWh m−3.
Palabras clave: Degradación de diclofenaco, electrodo de diamante dopado con boro, tratamiento de agua, electrooxidación, reactor FM01-LC.
Introduction
Over the last 15 years, pharmaceuticals have been receiving increasing attention as potential bioactive chemicals in the environment. They are considered as emerging pollutants in water bodies because they still remain unregulated or are currently undergoing a regularization process. In fact, it seems probable that most urban wastewater is contaminated with medicinal compounds. This affects the water quality and may constitute a potential risk for the ecosystems, the human and animal welfare at long term [1]. Some studies have reported the abundance of drugs in groundwater, urban wastewater plants, rivers and lakes around the world [1-14].
Many of these pharmaceutical compounds are not effectively removed by conventional wastewater treatment process. On the other hand, advanced oxidation processes have been largely investigated for their degradation [15-19]. Anodic oxidation with conductive diamond anode has presented many advantages as compared to other known chemical and photochemical processes. Some papers have reported the anodic oxidation treatment with BDD anode of wastewater contaminated with drugs [9-14]. A large quantity of hydroxyl radicals can be produced from water electrolysis on diamond surface, these radicals can oxidize the contaminants in the aqueous solution [16].
Some studies indicated that diclofenac can induce some adverse effects on aquatic life and in combination with other pharmaceuticals, present in water samples, the toxic effect can be considerably increased [8]. Removal of diclofenac from polluted and drinking water by advanced oxidation processes has been reported. Zhao et al. in (2009) examined the anodic oxidation degradation with BDD of 30 mg L−1 diclofenac in 0.1 M Na2SO4 and 0.1 NaCl without pH regulation, obtaining 72 % mineralization. These authors discussed that the diclofenac degradation is mediated by hydroxyl radical produced on BDD anode; besides the species as H2O2, O2 and active chlorine electrogenerated. On the other hand, Brillas et al. in (2010) performed a comparative study of anodic oxidation degradation with BDD and Pt anodes, with 175 mg L−1 diclofenac in 0.05 M Na2SO4 without pH regulation. Brillas et al. obtained more byproducts with Pt anode than BDD anode and also they reported that such diclofenac degradation is mediated by the action of peroxodisulfates coming from the oxidation of sulfates on both electrode surfaces.
This paper presents a study of the anodic oxidation of diclofenac in perchlorate media at neutral pH in a FM01-LC reactor equipped with BDD anode. The perchlorate medium was studied due to it do not produce byproducts from the electrolyte medium; therefore, the diclofenac degradation can be mediated by the action of the hydroxyl radical produced on BDD surface. The influence of mean linear flow velocity and current density on the performance of the diclofenac degradation is examined.
Experimental Details
The solution was prepared using diclofenac (150 mg L−1, 320 mg L−1 COD) in 0.5 M NaClO4 and pH 6.5. In this paper we used the above diclofenac concentration for research interest instead of resembling a typical concentration present in pharmaceutical wastewater or row wastewater, which ranged in other interval of concentration [20-21]. All the chemicals employed in this work were reactive grade.
Equipment
A potentiostat-galvanostat SP-150 BioLogic® with EC-Lab® software was used for all electrochemical experiments. The cell potential was measured with an AgilentTM high impedance multimeter.
COD and diclofenac analyses were performed using a dry-bath (Orbeco HELLIGE TR125008) and a UV-visible spectrophotometer (Perkin Elmer Lambda 35), respectively.
Microelectrolysis experiments
A 100-mL electrochemical cell, with a three electrode system was used for the microelectrolysis experiments. The rotating disk electrode (RDE) was a BDD disk with a geometric area of 0.018 cm2 exposed to the electrolyte. BDD RDE was provided by MetakemTM, with a thickness of 2-7 µm supported on Ti.
The potentials were measured vs. saturated calomel electrode (SCE) (Bio-Logic® model 002056RE-2B) and the counter electrode was a vitreous carbon rod. All the potential measurements shown in this work are presented with regard to standard hydrogen electrode (SHE).
Experiments in the FM01-LC
An exploded view of the cell that includes the turbulence promoter used within the cell channel is shown in Figure 1. In this work the spacer was 0.55 cm thick. We used a BDD anode, while a stainless steel plate was used as the cathode. BDD anode was 2-D (plate) provided by MetakemTM, with a thickness of 2-7 µm supported on Ti. Details on the FM01-LC cell characteristics are given in Table 1.
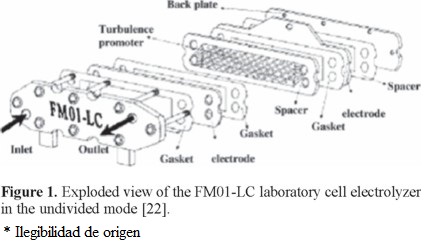

The undivided FM01-LC cell, with a single electrolyte compartment and the electrolyte flow circuit, is shown in Figure 2. The electrolyte was contained in a 2.5 L polycarbonate reservoir. A magnetically coupled pump of 1 hp was used. The flow rates were measured by a variable area glass rotameter from Flow-Meter, model F-44076LH-8. The electrolyte circuit was constructed from Master Flex tubing, C-Flex 6424-16, 0.5 inch diameter. The valves and the three way connectors were made of PVC.
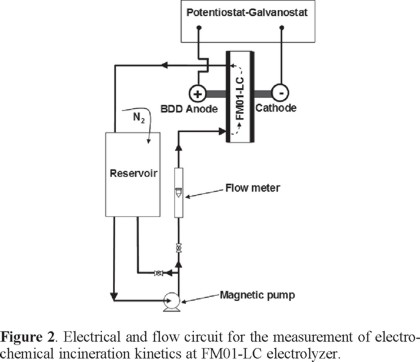
Methodology
Microelectrolysis tests
To stabilize the surface of the electrode and obtain reproducible results, a chronoamperogram was made to clean the BDD by anodic polarization in a 1 M HClO4 solution at 10 mA cm−2, for 30 minutes in the microelectrolysis cell. This treatment allows removing pollutants formed in the diamond [16].
Microelectrolysis tests were performed to determine the potential and current density limits, where diclofenac electrooxidation takes place. A typical three-electrode cell was used. Linear sweep voltammetry at 20 mV s−1 were performed from open circuit potential (OCP) (0.6 V vs. SHE) to the anodic potential limit of 3.0 V vs. SHE.
Electrooxidation of diclofenac was carried out in the FM01-LC cell equipped at different current density values of 10, 15 y 20 mA cm−2 and at different mean linear flow velocities comprised between 14.6 ≤ u ≤ 58.4 cm s−1. These values were determined from microelectrolysis studies and these will keep the electrode potential in the range 2.2 ≤ E ≤ 2.7 V vs. SHE, where the production of hydroxyl radicals was favored [16].
Electrooxidation evolution was estimated by COD analysis of samples taken at different times. The COD values were determined by closed reflux dichromate titration method [22]. Diclofenac concentration during electrochemical incineration was followed using UV-visible at λ=276 nm [8].
Results and discussion
Micro-electrolysis studies in the interface BDD-NaClO4
Figure 3 shows two typical sampled current density versus anodic potential curves (j vs. E) in a supporting electrolyte medium 0.5 M NaClO4, pH 6.5, T = 298 K in absence of diclofenac without rotation speed (Fig. 3a) and at 300rpm of BDD RDE (Fig. 3b). In addition, the inset of Figure 3 also shows a Tafel slope for Fig. 3a. From the analysis of this figure it was not observed any influence of hydrodynamics on anodic oxidation between the two curves (Fig. 3a, b); this behavior was very similar to that obtained in the presence of diclofenac (Fig. 3c), then, the anodic process corresponds to the oxidation of water which is not limited by mass transport.
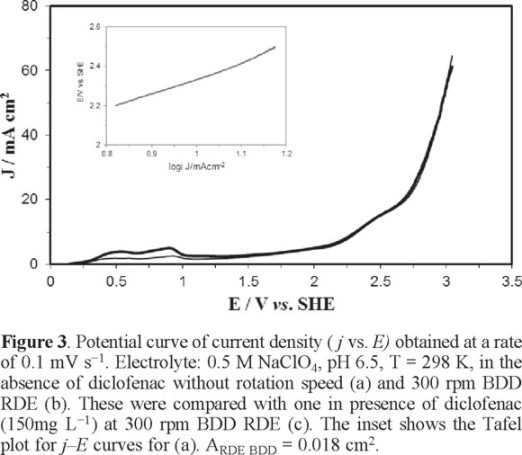
According to Nava et al. in (2007) at potentials between 2.3 ≤ E ≤ 2.75 V vs. SHE the formation of hydroxyl radicals, described by Equation (1), takes place [22].
BDD + H2O →BDD(•OH) + H+ + 1e− (1)
While at E > 2.7 V vs. SHE the anodic polarization curve presents a considerable increase in the slope, which corresponds to the oxygen evolution reaction [22].
H2O → 0.5O2 + 2H+ + 2e− (2)
The Tafel slope (inset of figure 3) was constructed to determine the potential range, where the formation of hydroxyl radical in the anode surface is favored. The construction of this curve was based on the linear polarization curve, Figure 3 (a). Tafel curve analysis was performed in the domain between 2.2 V <E <2.7 V vs SHE, yielding a slope of 0.810 V decade−1. This value is higher than the reported by Michaud et al. (2003) [17] and Nava et al. (2007) [22], who report values of 0.23 and 0.25 V decade−1, respectively. The difference of our Tafel slope can probably be associated with the manufacture of BDD, even when it was provided by the same supplier.
Electrooxidation of diclofenac in the FM01-LC using BDD electrode. Influence of the current density.
Figure 4 (a) and (b) show the normalized concentration and COD results obtained from experiments performed at different values of current density (10, 15 y 20 mA cm−2) and at constant u of 29.2 cm s−1, respectively. These current densities lead to remain the electrode potential between 2.3 ≤ E ≤ 2.75 V vs. SHE, where the formation of hydroxyl radicals is favored.

From the analysis of Figure 4 (a) a slow decrease in the concentration of diclofenac was observed during the first hour of electrolysis, then, an exponential decay is presented, achieving at 80% conversion to the four hours of the electrolysis. When the current density is increased to 15 mA cm−2, it is observed a rapid decay of the concentration and after 50 minutes a characteristic asymptote of concentration is presented. On the other hand, at 20 mA cm−2 the concentration depletion was slower than that obtained at 15 mA cm−2, contrary to that expected.
Figure 4(b) shows the normalized COD decay as function of time for the same set of electrolysis showed in Figure 4(a). The COD depletion was similar to that obtained in Figure 4(a). However, COD kinetic was lower to that obtained during diclofenac concentration decay owing to the degradation of by-products is slower than the degradation of diclofenac. Moreover, the typical electrolysis showed in Fig. 4 revealed that hydroxyl radical formation, responsible to the degradation of diclofenac, is favored at j of 10 and 15 mA cm−2; while at j of 20 mA cm−2 the oxygen evolution reaction start to appeared, diminishing the degradation rate.
In every case complete mineralization of the diclofenac is achieved. This last is attributed to the action of hydroxyl radical electrogenerated on the BDD owing to inert perchlorate medium avoids the apparition of other oxidants.
Electrooxidation of diclofenac in the FM01-LC reactor. Influence of mean linear fluid velocity.
Figure 5 (a) and (b) show the normalized concentration and COD results obtained from experiments performed at different mean linear flow velocities ranged between 14.6-58.4 cm s−1 at j of 15 mA cm−2. This current density was selected owing to the electrode potential will be in the range where large amounts of BDD(•OH) are produced favoring the degradation of diclofenac as previously discussed. In these figures, the normalized concentration and COD decreases with the electrolysis time at different mean linear flow rates.
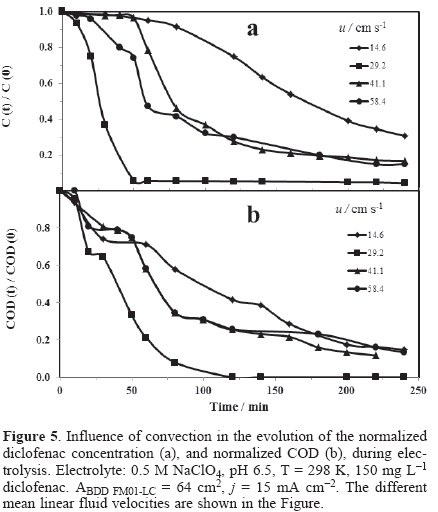
From the analysis of Figure 5 (a), at u = 14.6 cm s−1, it is observed a slow decrease in the concentration of diclofenac between 0 <t <120 minutes, consecutively a rapid decay appeared, achieving a conversion of 70% at four hours. Then, by increasing the linear flow velocity of 29.2 cm s−1 a rapid decrease in the concentration is observed, and after of 50 minutes a characteristic asymptote of concentration is presented. On the other hand, at u = 41.17 cm s−1 the concentration depletion was slower than that obtained at 29.2 cm s−1, contrary to that expected. For the last fluid rate (50.41 cm s−1) a slow descent is also observed in the concentration of diclofenac. This indicates that between 41.1 ≤ u ≤ 58.4 cm s−1, there is no dependence of the diclofenac kinetics with hydrodynamics owing to hydroxyl-organic contact at the BDD surface is minor [19].
Figure 5 (b) shows the normalized COD decay as function of time for the same electrolysis as shown in Figure 5 (a). The COD depletion was similar to that obtained in Figure 5 (a). However, COD kinetic was lower to that obtained during diclofenac concentration decay. For the mean linear flow velocities, 41.1 ≤ u ≤ 58.4 cm s−1, likewise there is not dependence of the diclofenac kinetics with hydrodynamics. The complete mineralization of diclofenac is only achieved at 29.2 cm s−1. It is important to mention that all of the electrolyses presented herein were developed in the undivided FM01-LC reactor, for which reason the degradation of organics may also involve reactions at the cathode.
Based on COD data obtained for all the previous electrolyses at their respective mean fluid velocity values, the average current efficiency (φ) was analyzed as a function of the percentage of COD removal. Figure 6 shows the current efficiency of mineralization at different linear flow velocities corresponding to the electrolysis shown in Figure 5. The average current efficiency was determined using the following expression [22]:
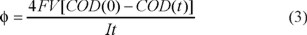

Where F is the Faraday's constant (96485 C mol−1), V is the volume of the solution (in cm3), COD(0) and COD(t) are the chemical oxygen demand (in mol cm−3) at times t = 0 (initial) and t (in s), respectively, and I is the applied current (in A).
From the analysis of Figure 6 it is observed that at mean linear fluid velocities of 14.6, 41.1 and 58.4 cm s−1 the current efficiency never reaches the theoretical. However, at u = 29.2 cm s−1, it is observed a current efficiency greater than the theoretical. The determination of percentage values over 100% suggests that the diclofenac oxidation byproducts help the degradation itself. It is believed that during the combustion of organic compounds the formation of organic radicals, which are relatively unstable, takes place [23]; the decomposition of such intermediates often leads to molecular cleavage and formation of subsequent intermediates with lower number of carbon atoms. These scission reactions continue rapidly until the conversion into carbon dioxide and water. A similar behavior was obtained by Nava during the incineration process of p and o-cresol [22] in the FM01-LC reactor equipped with a 2D BDD.
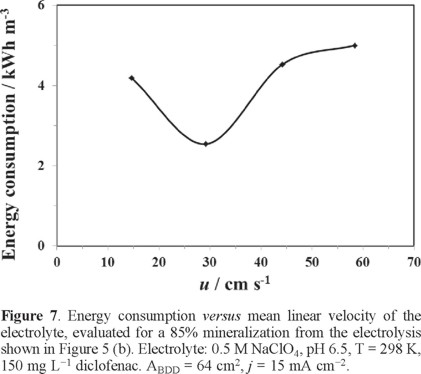
Curve in Figure 7 illustrate the energy consumption (Ec), evaluated at 85% COD removed, as a function of hydrodynamics during the electrolysis with the 2D BDD electrode at 15 mA cm−2 as a function of hydrodynamics. The following expression was employed for the estimation of Ec [22]:
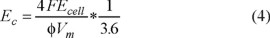
where Ecell is the cell voltage (in V), Vm is the molar volume (in cm3 mol−1) and 3.6 is a factor to express Ec in kWh m−3.
The analysis of Figure 7 shows that at 14 ≤ u ≤ 29 cm s−1, the energy consumption decreases, and at u > 29 cm s−1, it increases. The analysis of this curve shows that the energy consumption increased only slightly at the higher mean linear flow rates, and the variation of u did not have a significant effect on it.
Conclusions
Electrolyses in a FM01-LC reactor indicated that the oxidation of diclofenac was carried out via hydroxyl radicals formed by the oxidation of water in the BDD surface at current density ranged between 10-20 mA cm−2. The rate of diclofenac degradation and current efficiency of mineralization was not a function of the hydrodynamic, proving that diclofenac oxidation involves a complex mechanism.
The mineralization of diclofenac carried out at u = 29.2 cm s−1 achieved values of 100 %, with current efficiencies 78%, and energy consumption of 2.54 KWh m−3. The FM01-LC equipped with BDD anode improves space-time yield, allowing better interaction BDD(•OH) and organics, a phenomenon that increases organic mineralization efficiency.
Acknowledgments
Authors thank to CONACYT and CONCYTEG for the support via the project CONACYT-CONCYTEG GTO-2012-C04-195057. G. Coria acknowledge CONACYT for the given grant.
References
1. Sirés, I., Brillas, E. Environ. International. 2012, 40, 212-229. [ Links ]
2. Chen, H-C., Wang, P-L., Ding, W-H. Chemosphere 2008, 72, 863-869. [ Links ]
3. Bisceglia, J.K., Yua, T.J., Coelhanb, M., Bouwera, J.E., Robertsa, L.A. J. Chromatography A 2010, 1217, 558-564. (4) 36 [ Links ]
4. Verlicchi, P., Galletti, A., Petrovic, M., Barceló, D. J. Hydrology 2010, 389, 416-428. [ Links ]
5. Serrano, D., Suarez, S., Lema, J.M., Omil, F. Water Res. 2011, 45, 5323-5333. [ Links ]
6. Ranaa, D., Scheierb, B., Narbaitzb, R.M., Matsuuraa, T., Tabec, S., Saad, Y.J., Kailash, C. J. Membr. Sci. 2012, 409-410, 346-354. [ Links ]
7. Sui, Q., Wang, B., Zhao, W., Huang, J., Yu, G., Deng, S., Qiu, Z. Chemosphere 2012, 89, 280-286. [ Links ]
8. Zhao, X., Hou, Y., Liu, H., Qiang, Z., Qu, J. Electrochim. Acta. 2009, 54, 4172-4179. [ Links ]
9. Brillas, E., García, S., Skoumal, M., Arias, C. Chemosphere 2010, 79, 605-612. [ Links ]
10. Brillas, E., Sirés, I., Arias, C., Cabot, P.L., Centellas, F., Rodríguez, R.M., Garrido, J.A. Chemosphere 2005, 58, 399-406. [ Links ]
11. Ciríaco, L., Anjo, C., Correia, J., Pacheco, M.J., Lopes, A. Electrochim. Acta. 2009, 54, 1464-1472. [ Links ]
12. Guínea, E., Centellas, F., Brillas, E., Cañizares, P., Saéz, C., Rodrigo, M.A. Appl. Catal. B. Environ. 2009, 89, 645-650. [ Links ]
13. Sirés, I., Cabot, P. L., Centellas, F., Garrido, J.A., Rodríguez, R.M., Arias, C., Brillas, E. Electrochim. Acta. 2006, 52, 75-85. [ Links ]
14. Scheurell, M., Franke S., Shah R.M., Hühnerfuss H. Chemosphere 2009, 77, 870-876. [ Links ]
15. Verlicchi, P., Galletti, A., Petrovic, M., Barceló, D. J. Hydrology 2010, 389, 416-428. [ Links ]
16. Gherardini, L., Michaud, P.A., Panizza, M., Comninellis, Ch., Vatistas N. J. Electrochem. Soc. 2001, 148, D78-D82. [ Links ]
17. Michaud, P-A., Panizza, M., Ouattarra, L., Diaco, T., Foti, G., Comninellis, Ch. J. Appl. Electrochem. 2003, 33, 151-154. [ Links ]
18. Panizza, M., Ceriola, G. Electrochem. Acta. 2005, 51, 191-199. [ Links ]
19. Nava, J.L., Butrón, E., González, I. Environ. Eng. Manag. 2008, 18, 221-230. [ Links ]
20. Shi. X, Lefebvre.O, Kwang.K, Yong.H. Bioresource Technol. 2014, 153, 79-86. [ Links ]
21. Lagardea, F., Tusseau, M., Lessardb, P., He´duita, A., Dutropa, F., Mouchelc, J. Water Res. 2005, 39, 4768-4778. [ Links ]
22. Nava, J.L., Nuñez, F., González I. Electrochim. Acta. 2007, 52, 3229-3235. [ Links ]
23. Comninelis, C. Electrochim. Acta. 1994, 39, 1857-1862. [ Links ]














