Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.58 no.2 Ciudad de México abr./jun. 2014
Article
Zn(BH4)2/Ac2O/DOWEX(R)50WX4: A Novel System for Acylalation of Aldehydes
Davood Setamdideh
Department of Chemistry, College of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, Iran. d.setamdideh@iau-mahabad.ac.ir
Received March 4th, 2014
Accepted April 7th, 2014
Abstract
The acylalation of structurally different aldehydes has been performed by Zn(BH4)2/Ac2O/DOWEX(R)50WX4 as new system within 1-5 min at room temperature with excellent yields of the products (92-97%).
Key Words: Zn(BH4)2, Ac2O, DOWEX(R)50WX4, acylal, gem-diacetate, aldehyde.
Resumen
La acilalación de aldehídos estructuralmente diferentes ha sido realizada empleando Zn(BH4)2/Ac2O/DOWEX(R)50WX4 como un sistema novedoso, en 1-5 min a temperatura ambiente, con excelentes rendimientos de los productos (92-97%).
Palabras clave: Zn(BH4)2, Ac2O, DOWEX(R)50WX4, acilal, gem-diacetato, aldehído.
Introduction
Acylals have been used as starting materials for Diels-Alder [1], Grignard [2a], Barbier [2b], Prins [3], Knoevenagel [4a] and benzoin condensation reactions [4b]. Also, acylals were used in the synthesis of chrysanthemic acid [5a], sphingofungins E and F [5b] and utilized as cross linking reagents [6] in cellulose and cotton industry. However, the protection of carbonyl functional group of aldehydes is the main goal for the synthesis of acylals, because gem-diacetates are stable under critically controlled acidic, neutral and basic conditions [7].
Several reagents or catalysts such as amberlyst-15 [8], envirocat EPZ10 [9], montmorillonite [10], zeolites [11], nafion-H [12], FeSO4 [13], FeCl3 [14], AlCl3 [15], TMSCl-NaI [16], Sc(OTf)3 [17], I2 [18], NBS [19], PCl3 [20], H2SO4 [21], Cu(OTf)2 [22], LiBF4 [23], H2NSO3H [24], InCl3 [25], (NH4)2Ce(NO3)6 [26], LiOTf [27], Zn(BF4)2 [28], AlPW12O40 [29], ZrCl4 [30], Bi(NO3)3.5H2O [31], Bi(CF3SO3).4H2O [32], zirconium sulfohenyl phosphonat [33], GaCl3 [34], GaI3 [35], sulphated zirconia [36], poly(N,N'-dibromo-N-ethyl-benzene-1,3-disulfonamide) [PBBS] and N,N,N',N'-tetrabromobenzene-1,3-disulfon-amide [TBBDA] [37], saccharin sulfonic acid [38], zirconium hydrogen sulfate [39], Fe2(SO4)3·xH2O [40], erbium triflate [41], alum[KAl(SO4)2.12H2O] [42], sulfated zirconia [43], KHCO3 [44], H2SO4-silica [45], indium tribromide [46], HClO4-SiO2 [47], solid lithium perchlorate [48], zinc(II) perchlorate [49] and [Hmim] HSO4 [50] have been used for synthesis of gem-diacetates.
These methods are convenient but have some disadvantages such as long reaction times, harsh reaction conditions, use of strong acids, strictly reaction conditions and moisture sensitivity. Also, several of these catalysts are toxic, unavailable and costly. Thus, the research is still so much interest and we have investigated the acylalation of aldehydes in the presence of a reducing agent. So, in this context, we wish to introduce a fast and efficient method for the acylalation of a variety of aldehydes to their corresponding gem-diacetates using Ac2O and DOWEX(R)50WX4 in the presence of Zn(BH4)2.
Results and discussion
Recently, we have reported that DOWEX(R)50WX4 ion-exchange resin has been used for regioselective synthesis of oximes by NH2OH.HCl/DOWEX(R)50WX4 system [51], reduction of a variety of carbonyl compounds such as aldehydes, ketones, α-diketones, acyloins and α,β-unsaturated carbonyl compounds to their corresponding alcohols by NaBH4/DOWEX(R)50WX4 system [52], synthesis of cyanohydrins by NaCN/DOWEX(R)50WX4 [53] and reductive-amination of a variety of aldehydes and anilines by NaBH4/DOWEX(R)50WX4 [54].
On the other hand, Zn(BH4)2 is the modified borohydride agent which has better solubility in aprotic solvents such as THF, Et2O and DME. It is unique because of the better coordination ability of Zn2+ which is imparting selectivity in hydride-transferring reactions. We have developed the use of Zn(BH4)2 under new reducing system such as Zn(BH4)2/H2O [55], Zn(BH4)2/C [56], Zn(BH4)2/ZrCl4 [57], Zn(BH4)2/Al2O3 [58] and Zn(BH4)2/2NaCl [59]. In continuing our efforts for the development of using Zn(BH4)2 and DOWEX(R)50WX4, herein, we now wish to introduce Zn(BH4)2/Ac2O/DOWEX(R)50WX4 as new convenient system for efficient acylalation of aldehydes at room temperature.
For the selection of appropriate conditions, acylalation of benzaldehyde has been selected as model reaction. This reaction was performed with different amounts of Zn(BH4)2, Ac2O and DOWEX(R)50WX4 in different solvents (THF/Ac2O, Et2O/Ac2O, CH3CN/Ac2O, EtOAc/Ac2O, Ac2O) at room temperature as shown in Table 1.
Experiments show that the reaction was proceeded to give the highest yield in Ac2O. The optimization reaction conditions showed that the use of 1 molar equivalent of Zn(BH4)2 and 0.5 g of DOWEX(R)50WX4 in 1 mL Ac2O are the best conditions to complete the acylalation of benzaldehye (1 mmol) to gem-benzyldiacetate (Table 1, entry 4). Our observation revealed that the acylalation was completed within 1 min with 97% yield of product as shown in Scheme 1.

The efficiency of this protocol was examined by using structurally different aldehydes as shown in Table 2. In this Table, the entries 1-12 are simple aromatic aldehydes and entry 14 is a simple aliphatic aldehyde. Also, the entries 2-8 are aromatic aldehydes with electron-withdrawing groups while the entries 9-11 have electron-donating groups. The entry 13, corresponds to a α,β-unsaturated aldehyde. In this approach, the corresponding acylals were obtained in excellent yields (92-97%) and the reactions have been completed within 1-5 min as shown in Table 2. The products were characterized by the 1H-chemical shift of the CHs (Table 2, column 6) which appear around 6.8-8.0 ppm as a singlete (1H). Also the C=O stretching frequency in FT-IR spectrum of the products appears around 1748-1764 cm-1 (Table 2, column 7). For more characterization and verification of the products as shown in Table 2 (column 8), the melting points of the products have been measured and were compared with the literature.46-50
The ion-exchange resin DOWEX(R)50WX4 is insoluble in Ac2O. Therefore, the reactions take place under heterogeneous conditions. The influences of DOWEX(R)50WX4 and Zn(BH4)2 have been shown in Scheme 2. It seems that SO3H groups on DOWEX(R)50WX4 (as cation-exchange resin, strong acid) protonate the carbonyl group of aldehyde (Scheme 2, step I). Consequently, it is more susceptible for Ac2O attack (Scheme 2, step II). Also, the hydride attack from the Zn(BH4)2 promotes the formation of hydrogen gas which is slowly liberated in situ (Scheme 2, step III).

Also, the acylalation of cinnamaldehyde with 1 molar equivalent of Zn(BH4)2 and Ac2O (1 mL) in the presence of 0.5 g of DOWEX(R)50WX4 was carried out and the corresponding cinnamyl acylal was obtained within 1 min with 95% yield at room temperature (Table 2, entry 13).
Our attempt for the preparation of gem-diacetates from ketones was not successful using this system. Therefore, this system can act as chemoselective system for the acylalation of aldehydes over ketones. Thus, we have performed the acylalation of 1 molar equivalent of benzaldehyde in the presence of 1 molar equivalent of acetophenone under optimized reaction conditions (Table 3, entry 4) as shown in Scheme 3.
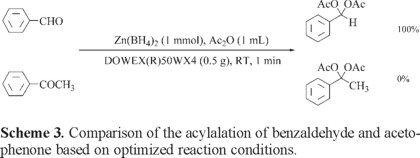
The chemoselectivity ratio for the acylalation of benzaldehyde with respect to acetophenone was 100%. The usefulness of chemoselectivity was further examined by the acylalation of benzaldehyde in the presence of other ketones as shown in Table 3.
The reusability of the catalyst has been checked by using recovered DOWEX(R)50WX4 for the acylalation of benzaldehyde under optimized reaction conditions (Table 1, entry 4). We have observed that the recovered DOWEX(R)50WX4 is not convenient for a second run without regeneration. However, after regeneration of DOWEX(R)50WX4 (stirred with HCl 5-10% for 30-60 min and then washed with distillated water), the acylalation reaction has been carried out as well as the first run as shown in Table 4.
Experimental
General. All substrates and reagents were purchased from commercially sources with the best quality and used without further purification. IR spectra were recorded on Perkin-Elmer FT-IR RXI and 1H NMR spectra were determined in a 300 MHz Bruker spectrometer. The products were characterized by their 1H NMR, 13C NMR or IR spectra and by comparison with authentic samples (melting points). All yields referred to isolated pure products. 1H NMR was applied for the purity determination of products and TLC for reaction monitoring over silica gel 60 F254 aluminum sheets.
The acylalation of benzaldehyde with Zn(BH4)2/DOWEX(R)50WX4/Ac2O system. A typical procedure
In a round-bottom flask (5 mL) equipped with a magnetic stirrer, a mixture of benzaldehyde (0.106 g, 1 mmol), Ac2O (1 mL) and DOWEX(R)50WX4 (0.5 g) was treated with Zn(BH4)2 (0.095 g, 1 mmol). The resulting reaction mixture was stirred at room temperature. After completion of the reaction within 1 min, the catalyst was filtered and washed with ethyl acetate (15 mL). The combined organic layers were washed with saturated NaHCO3 solution (3 × 10 mL), water (10 mL) and then dried over anhydrous Na2SO4. The solvent was removed on a rotary evaporator under reduced pressure to give 1,1-diacetoxy-1-phenylmethane (0.201 g, 97% yield). The product was characterized by 1H-NMR, 13C-NMR and FT-IR spectroscopy.
Spectral data for selected compounds
1,1-Diacetoxy-1-phenylmethane (Table 1, entry 1): 1H NMR (CDCl3): δ 2.14 (s, 6H), 7.41-7.43 (Ar, 3H), 7.52-7.55 (Ar, 2H), 7.69 (s, 1H); 13C NMR (CDCl3): δ 20.85, 89.67, 126.65, 128.58, 129.74, 135.40, 169.79; IR (KBr) ν = 3021, 1761, 1373, 1243, 1216, 1009, 767 cm-1.
1,1-Diacetoxy-1-(4-bromoyphenyl)methane (Table 1, entry 2): 1H NMR (CDCl3): δ 2.11 (s, 6H), 7.37-7.40 (Ar, 2H), 7.51-7.54 (Ar, 2H), 7.62 (s, 1H); 13C NMR (CDCl3): δ 20.78, 89.05, 123.90, 128.40, 131.76, 134.46, 168.64; IR (KBr) ν = 3025, 1764, 1373, 1236, 1208, 1070, 1012, 758 cm-1.
1,1-Diacetoxy-1-(4-nitrophenyl)methane (Table 1, entry 7): 1H NMR (CDCl3): δ 2.11 (s, 6H), 7.70 (Ar, 2H), 7.73 (s, 1H), 8.27 (Ar, 2H; 13C NMR (CDCl3): δ 20.74, 88.30, 123.84, 127.86, 141.87, 148.61, 158.55; IR (KBr) ν = 3033, 1759, 1367, 1239, 1208, 1067, 1007, 812 cm-1.
1,1-Diacetoxy-1-(4-methoxyphenyl)methane (Table 1, entry 9): 1H NMR (CDCl3): δ 2.06 (s, 6H), 3.75 (s, 3H), 6.70 (Ar, 2H), 7.43 (Ar, 2H), 7.61 (s, 1H); 13C NMR (CDCl3): δ 20.47, 55.08, 89.63, 113.79, 128.01, 131.86, 160.49, 168.72; IR (KBr) ν = 3018, 1748, 1600, 1367, 1259, 1160, 1027, 833 cm-1.
1,1-Diacetoxy-1-(2-metoxyphenyl)methane (Table 1, entry 10): 1H NMR (CDCl3): δ 2.10 (s, 6H), 3.82 (s, 3H), 6.90 (Ar, 1H), 6.98 (Ar, 1H), 7.34 (Ar, 1H), 7.49 (Ar, 1H), 8.03 (s, 1H); 13C NMR (CDCl3): δ 20.69, 55.52, 85.54, 110.88, 120.37, 123.72, 126.78, 130.84, 156.89, 168.44; IR (KBr) ν = 3016, 1761, 1602, 1372, 1220, 1005, 764 cm-1.
1,1-Diacetoxy-1-(4-methylphenyl)methane (Table 1, entry 11): 1H NMR (CDCl3): δ 2.12 (s, 6H), 2.38 (s, 3H), 7.23 (Ar, 2H), 7.43 (Ar, 2H), 7.66 (s, 1H); 13C NMR (CDCl3): δ 20.86, 21.28, 89.77, 126.60, 129.25, 132.59, 139.78, 168.80; IR (KBr) ν = 3033, 1759, 1367, 1239, 1067, 1039, 812 cm-1.
1,1-Diacetoxy-1-(cinnamyl)methane (Table 1, entry 13): 1H NMR (CDCl3): 2.13 (s, 6H), 6.22 (dd, J = 15 Hz, 6 Hz, 1H), 6.82 (d, J = 15 Hz, 1H), 7.27-7.44 (m, 5H), 7.37 (d, J =6 Hz, 1H); 13C NMR (CDCl3): δ 20.90, 89.71, 121.65, 127.00, 128.66, 128.83, 135.09, 135.60, 168.70; IR (KBr) ν = 3024, 1762, 1373, 1243, 1216, 1001, 756, cm-1.
Conclusions
In this research, we have shown that Zn(BH4)2/DOWEX(R)50WX4/Ac2O system is convenient method for the acylalation of a variety of aldehydes to their corresponding gem-diacetates in excellent yields. The acylalation reactions were carried out with 1 molar equivalents of Zn(BH4)2 and Ac2O (1 mL) in the presence of 0.5 g DOWEX(R)50WX4 at room temperature. High efficiency, shorter reaction times and easy work-up make to this new protocol attractive for the acylalation of aldehydes. Therefore, this new system could be a useful addition to the present methodologies.
Acknowledgment
The authors gratefully appreciated the financial support of this work with the grant No. 29/D/43755 by the research council of Islamic Azad University branch of Mahabad.
References
1. Banks, R. E.; Miller, J. A.; Nunn, M. J.; Stanley, P.; Weakley, T. J. R.; Ullah, Z. J. Chem Soc. Perkin Trans. 1 1981, 1096-1102. [ Links ]
2. (a) Sandberg, M.; Sydnes, L. K. Tetrahedron Lett. 1998, 39, 6361-6364. [ Links ] (b) Sydnes, L. K.; Sandberg, M. Tetrahedron 1997, 53, 12679-12690. [ Links ]
3. (a) Mowry, D. T. J. Am. Chem. Soc. 1950, 72, 2535-2537. [ Links ] (b) Merten, R.; Muller, G. Angew. Chem. 1962, 74, 866-871. [ Links ]
4. (a) Trost, B. M.; Vercauteran, J. Tetrahedron Lett. 1985, 26, 131-134. [ Links ] (b) Trost, B. M.; Lee, C. B.; Weiss, J. M. J. Am. Chem. Soc. 1995, 117, 7247-7248. [ Links ] (c) Sandberg, M.; Sydnes, L. K. Org. Lett. 2000, 2, 687-689. [ Links ]
5. (a) Kula, J. Pol. Pat. PL 143, 824 1988; (CA 112, 216290y). [ Links ] (b) Trost, B. M.; Lee, C. J. Am. Chem. Soc. 2001, 123, 12191-12201. [ Links ]
6. Frick, J. G.; Harper, R. J. Jr. J. Appl. Polym. Sci.1984, 29, 1433-1447. [ Links ]
7. (a) Greene, T. W.; Wuts, P. G. M. Protective Groups in Organic Synthesis, 3nd Ed., Wiley-VCH, New York, 1999, p 306. [ Links ] (b) Gregory, M. J. J. Chem. Soc. (B) 1970, 1201-1207. [ Links ]
8. Reddy, A.V.; Ravinder, K.; Reddy, V. L. N.; Ravikanth, V.; Yenkateswarlu, Y. Synth.Commun. 2003, 33, 1531-1536. [ Links ]
9. Bandgar, B. P.; Makone, S. S.; Kulkarni, S. P. Monatsh. Chem. 2000, 131, 417-420. [ Links ]
10. Li, T. S.; Zhang, Z. H.; Gao, Y. J. Synth. Commun. 1998, 28, 4665-4671. [ Links ]
11. (a) Kumar, P.; Hegde, V. R.; Kumar, T. P. Tetrahedron Lett. 1995, 36, 601-602. [ Links ] (b) Pereira, C. B.; Gigante, M. J.; Marcelo-Curto, H.; Carreyre, G.; Perot, G.; Guisnet, M. Synthesis 1995, 1077-1078. [ Links ] (c) Ballini, R.; Bordoni, M.; Bosica, G.; Maggi, R.; Sartori, G. Tetrahedron Lett. 1998, 39, 7587-7580. [ Links ]
12. Olah, G. A.; Mehrotra, A. K. Synthesis 1982, 962-963. [ Links ]
13. Jin, T. S.; Du, G. Y.; Li, T. S. Ind. J. Chem., Sec. B 1998, 939-940. [ Links ]
14. Wang, C.; Li. M. Synth. Commun. 2002, 32, 3469-3473. [ Links ]
15. Gowravaram, S.; Abraham S.; Ramalingam, T.; Yadav, J. S. J. Chem. Res. (S) 2002, 3, 144-146. [ Links ]
16. Deka, N.; Borah, R.; Kalita, D. J.; Sarma, J. C. J. Chem. Res.(S) 1998, 94-95. [ Links ]
17. Agarwal, V. K.; Fonquerna, S. S.; Vennall, G. P. Synlett 1998, 849-850. [ Links ]
18. Deka, N.; Kalita, D. J.; Borah, R.; Sarma, J. C. J. Org. Chem. 1997, 62, 1563-1564. [ Links ]
19. Karimi, B.; Seradj, H.; Ebrahimian, R. G. Synlett 2000, 623-624. [ Links ]
20. Michie, J. K.; Miller, J. A. Synthesis 1981, 824-824. [ Links ]
21. Gregory, M. J. J. Chem. Soc. B 1970, 1201-1207. [ Links ]
22. Chandra, K. L.; Saravanan, P.; Singh, V. K. Synlett 2000, 359-360. [ Links ]
23. Sumida, N.; Nishioka, K.; Sato, T. Synlett 2001, 1921-1922. [ Links ]
24. Jin, T. S.; Sun, G.; Li, Y. W.; Li, T. S. Green Chem. 2002, 4, 255-256. [ Links ]
25. Yadav, J. S.; Reddy, B. V. S.; Srinivas, Ch. Synth.Commun. 2002, 32, 2169-2174. [ Links ]
26. Roy, S. C.; Banerjee, B. Synlett 2002, 1677-1678. [ Links ]
27. Karimi, B.; Maleki, J. J. Org. Chem. 2003, 68, 4951-4954. [ Links ]
28. Ranu, B. C.; Dutta, J.; Das, A. Chem. Lett. 2003, 32, 366-367. [ Links ]
29. Firouzabadi, H.; Iranpoor, N.; Nowrouzi, F.; Amani, K. Tetrahedron Lett. 2003, 44, 3951-3954. [ Links ]
30. Smitha, G.; Reddy, Ch. S. Tetrahedron 2003, 59, 9571-9576. [ Links ]
31. Aggen, D. H.; Arnold, J. N.; Hayes, P. D.; Smoter, N.; Mohan, R. S. Tetrahedron 2004, 60, 3675-3679. [ Links ]
32. Carrigan, M. C.; Eash, K. J.; Oswald, M. C.; Mohan, R. S. Tetrahedron Lett. 2001, 42, 8133-8135. [ Links ]
33. Curini, M.; Epifano, F.; Marcotullio, M. C.; Rosati, O.; Nocchetti, M. Tetrahedron Lett. 2002, 43, 2709-2711. [ Links ]
34. Kumar, S.; Saini, A.; Sandhu, J. A. Arkivoc 2007, 14, 27-33. [ Links ]
35. Sun, P.; Hu, Z. J. Chem. Res. 2005, 10, 659-660. [ Links ]
36. Palacios-Grijalva, L. N.; Cruz-González, D. Y.; Lomas-Romero, L.; González-Zamora, E.; Ulibarri, G.; Negrón-Silva, G. E. Molecules 2009, 14, 4065-4078. [ Links ]
37. Ghorbani-Vaghei, R.; Amiri, M.; Moshfeghifar, N.; Veisi, H.; AkbariDadamahaleh, S. J. Iran. Chem. Soc. 2009, 6, 754-760. [ Links ]
38. Shirini, F.; Mamaghani, M.; Mostashari-Rad, T.; Abedini, M. Bull. Korean Chem. Soc. 2010, 31, 2399-2401. [ Links ]
39. Mirjalili, B. F.; Zolfigol, M. A.; Bamoniri, A.; Sheikhana, N. J. Chin. Chem. Soc. 2006, 53, 955-959. [ Links ]
40. Zhang, X.; Li, L.; Zhang, G. Green Chemistry 2003, 5, 646-468. [ Links ]
41. Dalpozzo, R.; Nino, A. D.; Maiuolo, L.; Nardi, M.; Procopio, A.; Russo, B.; Tagarelli, A. Arkivoc 2006, 6, 181-189. [ Links ]
42. Shelke, K.; Sapkal, S.; Kategaonkar, A.; Shingate, B.; Shingare, M . S. S. Afr. J. Chem. 2009, 62, 109-112. [ Links ]
43. Negrón, G. E.; Palacios, L. N.; Angeles, D.; Lomas, L.; Gaviño, R. J. Mex. Chem. Soc. 2005, 49, 252-256. [ Links ]
44. Heravi, M. M.; Bakhtiari, K.; Taheri, S.; Oskooie, H. A. Green Chem. 2005, 7, 867-869. [ Links ]
45. Pourmosavi, S. A.; Zinati, Z. Turk. J. Chem. 2009, 33, 385-392. [ Links ]
46. Yin, L.; Zhang, Z. H.; Wang, Y. M.; Pang, M. L. Synlett 2004, 10, 1727-1730. [ Links ]
47. Khan, A. T.; Choudhury, L. H.; Ghosh, S. J. Mol. Catal. A: Chem. 2006, 255, 230-235. [ Links ]
48. Ziyaei, A.; Azizi, N.; Saidi, M. R. J. Mol. Catal. A: Chem. 2005, 238, 138-141. [ Links ]
49. Kumar, R.; Thilagavathi, R.; Gulhane, R.; Chakraborti, A. K. J. Mol. Catal. A: Chem. 2006, 250, 226-231. [ Links ]
50. Hajipour, A. A.; Khazdooz, L.; Ruoho, A. E. Catal. Commun. 2008, 9, 89-96. [ Links ]
51. Setamdideh, D.; Khezri, B.; Esmaeilzadeh, S. J. Chin. Chem. Soc. 2012, 59, 1119-1124. [ Links ]
52. Setamdideh, D.; Khezri, B.; Alipouramjad, A. J. Chin. Chem. Soc. 2013, 60, 590-596. [ Links ]
53. Sofighaderi, S.; Setamdideh, D. Orient. J. Chem. 2013, 29, 1135-1137. [ Links ]
54. Setamdideh, D.; Sepehraddin, F. J. Mex. Chem. Soc. 2014, 58, 22-26. [ Links ]
55. Setamdideh, D.; Khezri, B.; Rahmatollahzadeh, M.; Aliporamjad, A. Asian J. Chem. 2012, 8, 3591-3596. [ Links ]
56. Setamdideh, D.; Rahmatollahzadeh, M. J. Mex. Chem. Soc. 2012, 56, 169-175. [ Links ]
57. Kamari, R.; Setamdideh, D. Orient. J. Chem. 2013, 29, 497-499. [ Links ]
58. Setamdideh, D.; Khezri, B.; Rahmatollahzadeh, M. J. Serb. Chem. Soc. 2013, 78, 1-13. [ Links ]
59. Setamdideh, D.; Khaledi, L. S. Afr. J. Chem. 2013, 66, 150-157. [ Links ]














