Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.58 n.2 Ciudad de México Apr./Jun. 2014
Article
Synthesis, Spectroscopic Characterization, Thermal Analysis and Antibacterial Activity of Ni(II), Cu(II) and Zn(II) Complexes with Schiff bases Derived from β-Diketones
Razieh Ahmadzadeh,1 Mohammad Azarkish,2 and Tahereh Sedaghat3*
1 Department of Chemistry, Science and Research Branch, Islamic Azad University, Khouzestan, Iran.
2 Department of Chemistry, Shoushtar Branch, Islamic Azad University, Shoushtar, Iran.
3 Department of Chemistry, College of Sciences, Shahid Chamran University, Ahvaz, Iran. tsedaghat@scu.ac.ir
Received July 19th, 2013
Accepted February 28th, 2014
Abstract
Five transition metal complexes, [CuLa] (1), [NiLa] (2), [ZnLa] (3), [CuLb] (4) and [NiLb].EtOH (5) have been synthesized from reaction of Ni(II), Cu(II) and Zn(II) acetate salts with two Schiff bases, 3-(2-hydroxy-5-methylphenylamino)-1,3-diphenylprop-2-en-1-one (H2La) and 3-(2-hydroxy-5-methylphenylimino)-1-phenylbuten-1-one (H2Lb). On the basis of analytical and spectral data, Schiff base is coordinated to metal as tridentate dianionic ligand via phenolic and enolic oxygens and imine nitrogen. Thermal decomposition of the complexes has been studied by thermogravimetry. The in vitro antibacterial activity of Schiff bases and their complexes has been evaluated against Gram-positive (Bacillus subtilis and Staphylococcus aureus) and Gram-negative (Escherichia coli and Pseudomonas aeruginosa) bacteria and compared with the standard drugs.
Key words: Schiff base, transition metal, thermogravimetry, antibacterial activity, diketones.
Resumen
Se sintetizaron cinco complejos de metales de transición, [CuLa] (1), [NiLa] (2), [ZnLa] (3), [CuLb] (4) y [NiLb].EtOH (5), mediante la reacción de iones Ni(II), Cu(II), Zn(II) con dos compuestos tipo base Schiff, 3-(2-hidroxi-5-metilfenilamino)-1,3-difenilprop-2-en-1-ona (H2La) y 3-(2-hidroxi-5-metilfenilimino)-1-fenilbuten-1-ona (H2Lb). Los resultados de los análisis y los datos espectrales obtenidos revelaron que en la base Schiff actúa como un ligando tridental dianiónico, coordinando al ion metálico mediante oxígenos fenólico y enólico y mediante nitrógeno del grupo imino. La descomposición térmica de los complejos se estudió por termogravimetría. La actividad antibacteriana de los dos ligandos y de los cinco complejos ha sido evaluada en ensayos in vitro utilizando cepas de bacterias Gram-positivas (Bacillus subtilis, Staphylococcus aureus) y de bacterias Gram-negativas (Escherichia coli, Pseudomonas aeruginosa) comparando los resultados con los obtenidos para fármacos estándar.
Palabras clave: Base Schiff, metal de transición, termogravimetría, actividad antibacteriana, dicetonas.
Introduction
Schiff bases are among the most widely used ligands and play an important role in metal coordination chemistry due to facile synthesis, strong coordination abilities, remarkable versatility and good biological activities [1]. Many of these compounds exhibit tautomeric rearrangements and receive interest due to several applications in optical recording technology and molecular electronics [2, 3]. The research field dealing with metal complexes of Schiff bases is very broad because of their potential interest for a number of areas including bioinorganic chemistry, catalysis and electrochemistry [4-7]. The development in the field of biological inorganic chemistry has increased the interest in Schiff base complexes. A large number of Schiff bases and their metal complexes have been investigated due to their interesting biological properties such as their ability to reversibly bind oxygen, biological modeling applications and antibacterial, antifungal, anticancer, and herbicidal activities [8-13]. In many cases, when Schiff bases administered as their metal complexes, the biological activity of these complexes is enhanced in comparison to the free ligand [14-17].
Recently we have reported synthesis and antibacterial activities of several Schiff bases derived from β-diketones and their metal complexes [16-20]. These type of Schiff bases contain the N(sp3)-C(sp2)-C(sp2) -C(sp2)=O(sp2) fragment which is bounded by strong hydrogen bonds (N-H...O or N. . .H-O). These compounds are of biological interest, play as synthetic intermediates in organic reactions and act as sensor materials [2, 21]. As an extension of our investigation, herein we report synthesis, characterization, thermal behavior and antibacterial activities of Ni(II), Cu(II) and Zn(II) complexes with two Schiff bases, 3-(2-hydroxy-5-methylphenylamino)-1,3-diphenylprop-2-en-1-one (H2La) and 3-(2-hydroxy-5-methylphenylimino)-1-phenylbuten-1-one (H2Lb) (Fig. 1.).
Results and Discussion
The Schiff bases, 3-(2-hydroxy-5-methylphenylamino)-1,3-diphenylprop-2-en-1-one (H2La) and 3-(2-hydroxy-5-methylphenylimino)-1-phenylbuten-1-one (H2Lb), have been prepared from reaction of 2-amino-4-methylphenol with dibenzoyl methane or benzoylacetone, respectively. In these reactions only 1:1 condensation was occurred even when an excess of the amine was used. Structural formula for these Schiff bases in three tautomeric forms is given in Fig. 1. According to previous evidences, keto-amine tautomeric form (III) is preferred in solid state [16-19, 22]. The new complexes 1-5 were synthesized by reaction Cu(II), Ni(II) and Zn(II) acetate salts with corresponding Schiff base ligands in ethanol. Acetate anion acts also as a base to force the deprotonation of the ligand. The new complexes were investigated by elemental analysis, spectroscopic methods and thermogravimetric analysis. Our attempts to grow single crystals suitable for X-ray crystallography were abortive.
Spectroscopic studies
Fig. 2 show the IR spectra of ligands and their complexes. In the infrared spectrum of free Schiff bases the stretching vibration of the OH/NH appears at lower frequency and overlap with the ν(C-H) in the range of 3000-3200 cm-1 due to the inter-/intra-molecular hydrogen bonding. This broad band is completely absent in the spectra of all complexes supporting deprotonation of the ligand during coordination. In the infrared spectra of all complexes no band is observed in the region 3300-3500 cm-1 attributable to the stretching vibration of hydrated or coordinated water. In the spectrum of free ligands a band observed in 1615 cm-1 assigned as a perturbed carbonyl stretching with the frequency lowering from a free carbonyl ascribed to conjugation and hydrogen bonding in keto-amine form (III). In the IR spectra of complexes, this band shifts to lower frequency providing evidence of participation of oxygen in bonding with metal and so weakening of C=O bond. A band at 1583-1590 cm-1 in the spectra of complexes assigned to C=C/C=N bond indicates the anionic ligand is coordinated to metal in the tautomeric form II. The appearance of new bands in the IR spectra of the complexes in the region 453-585 cm-1, which may be assigned to ν(M-N) and ν(M-O), supports the bonding of nitrogen and oxygen to the metal ion [23-25].
In the electronic spectra of complexes, d-d bands were not observed because of low solubility of compounds in DMSO and small molar absorption coefficient of these forbidden transitions. Therefore all appeared bands in electronic spectra result from the overlap of π→π* transitions mainly localized within the imine chromophore and the ligand to metal charge-transfer (LMCT) transition from the lone pairs of the oxygen to the M(II) ions [26]. 1H NMR spectrum for Zn(II) complex was not recorded because of low solubility of this complex in common NMR solvents.
Thermogravimetric analyses
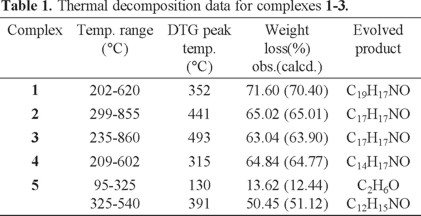
The thermogravimetric analyses of complexes have been studied from ambient temperature up to 1000 °C under a N2 atmosphere. The thermogravimetric and derivative thermogravimetric (TG and DTG) analysis curves for 1-5 are represented in Fig. 3. Thermal behaviors of complexes have been summarized in Table 1. The results show the presence of a ligand molecule per metal ion and confirm the formulae suggested from the analytical data. The absence of weight loss up to 200 °C indicates that there is no hydrated water molecule in the crystalline solid in 1-4 complexes, while complex 5 losses one mol EtOH in this range. Complex 1 experience a weight loss between 202-620 °C which can be interpreted as loss of the greatest part of the ligand. Complex 2 shows a drastic mass loss in one step within temperature range 299-855 °C which attributes to the decomposition of the ligand and the removal of the C17H17NO. Complex 3 also shows one-step decomposition between 235-860 °C corresponds to similar mass loss. The thermal degradation of complex 4 corresponds to the loss of C14H15NO in one step. In the first thermal degradation step of 5, ethanol is eliminated and the second step (325-540 °C) evolves C12H15NO. In all cases the residues are carbon and metal oxide. In general, complexes 1-5 exhibit good thermal stability and the Ni(II) complexes are more stable than the others.
Biological studies
The in vitro antibacterial activities of Schiff bases and their complexes were studied along with two standard antibacterial drugs, viz, Nalidixic acid and Vancomycin. The microorganisms used in this work include Bacillus subtilis and Staphylococcus aureus (as Gram-positive bacteria) and Escherichia coli and Pseudomonas aeruginosa (as Gram-negative bacteria). The results are presented in Table 2. The antibacterial activity of compounds is due to either bactericide effects (killing the bacteria) or bacteriostatic effects (inhibiting multiplication of bacteria by blocking their active sites). It may be postulated that antibacterial compounds deactivate various cellular enzymes, which play a vital role in various metabolic pathways of these organisms. It has also been proposed that the ultimate action of the antibacterial agent is the denaturation of one or more proteins of the cell, which, as a result, impairs normal cellular processes [27]. However, it is apparent that permeability across the bacterial cell wall is necessary for the effectiveness of the biocide compounds; therefore any factor which facilitates microorganism membrane crossing may enhance the activity. Comparing the biological activity of the Schiff bases, complexes and standard drugs, indicate that all complexes exhibit more inhibitory effects than the parent ligands against bacterial strains. This enhanced antibacterial activity may be due to electron delocalization over the whole chelate ring upon complexation. Such chelation increases the lipophilicity and enhances the permeation through the lipid layer of the cell membrane (chelation theory) [28-32]. It is apparent that complexes were more toxic towards Gram-positive than Gram-negative bacteria strains. The reason is the difference in the structures of the cell walls. Lipopolysacharides form an outer lipid membrane and contribute to the complex antigenic specificity of Gram-negative cells [33].
Conclusion
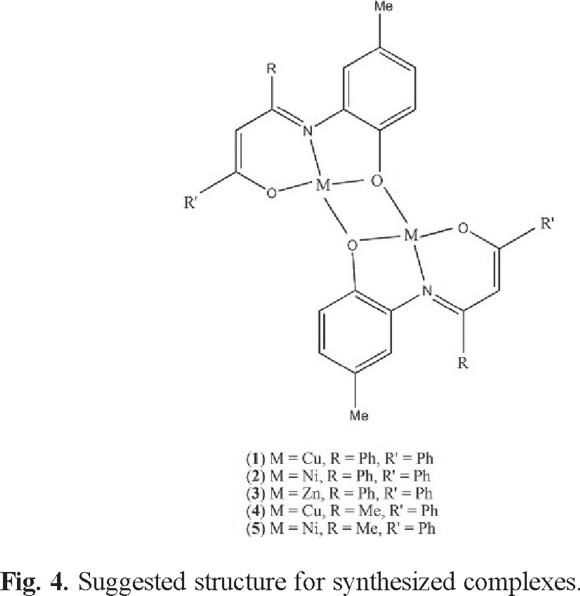
On the basis of above analytical and spectral data, in all complexes the Schiff base is deprotonated and coordinated to metal ion as tridentate dianionic ligand via imine nitrogen and phenolic and enolic oxygens. Thermal and elemental analysis data show no water molecules in the formula of complexes. It is suggested that the coordination sphere of complex is completed by phenolic oxygen atom of another molecule generating a dimer. Similar dimeric structures have been reported earlier for Schiff base complexes [19, 28, 34-38]. According to previous report [20], the interaction occurs with the oxygen atom forming a part of the five-membered heterocycle that can be attributed to the increased "s" character at the five-membered ring [37]. Therefore, we suggest a dimeric structure for synthesized complexes with a distorted square planar geometry around metal ions (Fig. 4). On the basis of thermal analysis data, the complexes have been found to be thermally stable. All complexes exhibited good activities against gram positive bacteria and have a potential to be used as drugs.
Experimental
Materials and methods
All starting materials were purchased from Merck Company and used as received. All solvents were of reagent grade and used without further purification. IR spectra were obtained using a FT BOMEM MB102 spectrophotometer. The 1HNMR spectra were recorded in DMSO-d6 with a Bruker 400 MHz Avance Ultrashield spectrometer. Thermogravimetric analyses (TGA) were carried out using a Perkin-Elmer Diamond thermal analysis. The heating rates were controlled at 5 °C min-1 under a nitrogen atmosphere with a 150 ml/min flow rate and the weight loss was measured from ambient temperature up to 1000 °C.
Synthesis of Schiff bases
3-(2-hydroxy-5-methylphenylamino)-1,3-diphenylprop-2-en-1-one (H2La) H2La was prepared from reaction of 2-amino-4-methylphenol and dibenzoylmethane as the method reported earlier [19], herein some 1HNMR data have been revised. λmax (nm, DMSO): 400, 380, 360; IR (KBr,cm-1): 1589, ν(C=C); 1615, ν(C=O), 3000-3150 br, ν(C-H), ν(O-H)/ν(N-H). 1H NMR (DMSO-d6): δ 1.86 (s, 3H, CH3), 6.08 (d, 4J = 1.3 Hz, 1H, H14), 6.11 (s, 1H, H6), 6.65 (dd, 3J = 8.0, 4J = 1.5 Hz, 1H, H13), 6.76 (d, 3J = 8.1 Hz, 1H, H12), 7.39-7.55 (m, 8H, H2,3,4,7,8,910,11), 7.99 (d, 3J = 7.2 Hz, 2H, H1,5), 9.79 (s, 1H, OHphenolic), 12.61 (s, 1H, NH/OHenolic).
3-(2-hydroxy-5-methylphenylimino)-1-phenylbuten-1-one (H2Lb)
An ethanolic solution (20 ml) of 2-amino-4-methylphenol (1.232 g, 10 mmol) was added to a solution of benzoylacetone (1.622 g, 10 mmol) in ethanol (20 ml). This solution was refluxed for 8 h. The Schiff base precipitates after standing 24 h at rt. The product was filtered off and washed with ethanol (2 × 5 mL). Yield: 1.944 g (73%); m.p. 148-150 °C; λmax (nm, DMSO): 368; IR (KBr,cm-1): 1589, ν(C=C); 1615, ν(C=O), 3000-3150 br, ν(C-H), ν(O-H)/ν(N-H). 1H NMR (DMSO-d6): δ 2.15 (s, 3H, CH3), 2.23 (s, 3H, CH3), 6.03 (s, 1H, H6), 6.87 (m, 2H, H8,9), 7.08 (s, 1H, H7), 7.45 (m, 2H, H2,3,4), 7.92 (d, 3J = 6.4 Hz, 2H, H1,5), 9.80 (s, 1H, OHphenolic), 12.82 (s, 1H, NH/OHenolic).
General procedure for synthesis of complexes
The Schiff base (0.5 mmol) in EtOH (5 ml) was added to an equimolar amount of Ni(OAc)2.4H2O, Cu(OAc)2.H2O or Zn(OAc)2.2H2O in EtOH (5 ml). The solution was refluxed about 4 h for 1, 2 and 3, whereas 8 h for 4. Complex 5 was precipitate after 3 h stirring in rt. The products were filtered, washed with MeOH and dried in vacuum on CaCl2. Our attempts to synthesize [ZnLb] were unsuccessful.
[CuLa] (1): Yield: 0.249 g (64%); λmax (nm, DMSO): 317, 436; IR (KBr, cm-1): ν(C=N) / (C=C), 1584; ν(C-O), 1553; ν(Cu-N), 571; ν(Cu-O), 455; Anal. Calcd. for C22H17NO2Cu: C, 67.53; H, 4.35; N, 3.56%. Found: C, 67.97; H, 4.13; N, 3.94%.
[NiLa] (2): Yield: 0.236 g (61%); λmax (nm, DMSO): 458, 300; IR (KBr, cm-1): ν(C=N)/(C=C), 1584; ν(C-O), 1563; ν(Ni-N), 556; ν(Ni-O), 455; Anal. Calcd. for C22H17NO2Ni: C, 68.39; H, 4.40; N, 3.62%. Found: C, 68.26; H, 4.40; N, 3.52%.
[ZnLa] (3): Yield: 0.231 g (62%); λmax (nm, DMSO): 351, 452; IR (KBr, cm-1): ν(C=N)/(C=C), 1583; ν(C-O), 1563; ν(Zn-N), 562; ν(Zn-O), 464; Anal. Calcd. for C22H17NO2Zn: C, 67.21; H, 4.32; N, 3.56%. Found: C, 66.79; H, 4.37; N, 3.51%.
[CuLb] (4): Yield: 0.280 g (86%); λmax (nm, DMSO): 435, 360; IR (KBr, cm-1): ν(C=N)/(C=C), 1583; ν(C-O), 1556; ν(Cu-N), 585; ν(Cu-O), 459; Anal. Calcd. for C17H15NO2Cu: C, 62.03; H, 4.56; N, 4.26%. Found: C, 62.15; H, 4.48; N, 4.3%.
[NiLb] (5): Yield: 0.243 g (75%); λmax (nm, DMSO): 439, 310; IR (KBr, cm-1): ν(C=N)/(C=C), 1592; ν(C-O), 1567; ν(Ni-N), 572; ν(Ni-O), 453; Anal. Calcd. for C19H21NO3Ni: C, 61.67; H, 5.68; N, 3.78%. Found: C, 61.45; H, 5.70; N, 3.91%.
Antibacterial tests
The in vitro antibacterial activity of ligands and their corresponding complexes was investigated against the standard strains of two Gram-positive (Bacillus subtilis ATCC 12711 and Staphylococcus aureus ATCC 6538) and two Gram-negative (Escherichia coli ATCC 35218 and Pseudomonas aeruginosa ATCC 27853) bacteria. In order to compare the results, Nalidixic acid (30 mg/disc) and Vancomycin (30 mg/disc) were used as standard antibacterial drugs. Determination of the antibacterial activity was carried out by paper-disc diffusion method. The compounds were dissolved in DMSO at 100, 200 and 400 μg/ ml concentration. Muller Hinton broth was used for preparing basal media for the bioassay of the organisms. A lawn culture from 0.5 MacFarland suspension of each strain was prepared on Muller Hinton agar. Blank paper discs (6.4 mm diameter) were saturated with a solution of test compounds and placed on the surface of the agar plates. On one paper disc only DMSO was poured as a control. The plates were incubated at 37 °C for 24 h. The inhibition zone diameters around each disc were measured in mm.
Acknowledgment
Support of this work by Shahid Chamran University, Ahvaz, Iran and Science and Research Branch, Islamic Azad University, Ahvaz, Iran is gratefully acknowledged.
References
1. Hernandez-Molina, R.; Mederos, A. In Comprehensive Coordination Chemistry; McCleverty, J. A.; Meyer, T.J., Ed.; 2nd ed.; Elsevier, 2005; Vol 1, Chapter 19. [ Links ]
2. Roderiguez, M.; Santillan, R.; Lopez, Y.; Farfan, N.; Rarra, V.; Nakatani, K.; Garciabaez, E. V.; Supramol. Chem. 2007, 19, 641-653. [ Links ]
3. Minkin, V. I.; Tsukanov, A. V.; Dubonosov, A. D.; Bren, V. A.; J. Mol. Struct. 2011, 998, 179-191. [ Links ]
4. Hamilton, D.E.; Drago, R.S.; Zombeck, A.; J. Am. Chem. Soc. 1987, 109, 374-379. [ Links ]
5. You, J.; Liu, B.; Wang, Y. J.; Chin. Chem. Soc. 2009, 56, 1010-1017. [ Links ]
6. Akhond, M.; Najafi,M. B.; Sharghi, H.; Tashkhourian, J.; Naiemi, H.; J. Chin. Chem. Soc. 2007, 54, 331-337. [ Links ]
7. Sönmez, M.; Celebi, M.; Berber, I.; Eur. J. Med. Chem. 2010, 45, 1935-1940. [ Links ]
8. Chen, D.; Martel, A.E.; Inorg. Chem. 1987, 26, 1026-1030. [ Links ]
9. El-halim, H. F. A.; Omar, M.M.; Mohamed, G. G.; Spectrochim. Acta Part A 2011, 78, 36-44. [ Links ]
10. Abou-Hussen, A. A.A.; Linert, W.; Syn. React. Inorg. Metal-Org. Chem. 2009, 39, 570-599. [ Links ]
11. Silva, C. M.; Silva, D. L.; Modolo, L. V.; Alves, R. B.; Resende, M. A.; Martins, C.V.B.; Fatima, J. Adv. Res. 2011, 2, 1-8. [ Links ]
12. Venkatesan, K.; Satyanarayana, V. S. V.; Sivakumar, A.; J. Chin. Chem. Soc. 2011, 58, 583-589. [ Links ]
13. Bagihalli, G. B.; Avaji, P. G.; Patil, S. A.; Badami, P. S.; Eur. J. Med. Chem. 2008, 43, 2639-2649. [ Links ]
14. Collinson, S. R.; Fenton, D. E.; Coord. Chem. Rev. 1996, 148, 19-40. [ Links ]
15. Saxena, A. K.; Huber, F.; Coord. Chem. Rev. 1989, 95, 109-123. [ Links ]
16. Sedaghat,T.; Monajemzadeh, M.; Motamedi, H.; J. Coord. Chem. 2011, 64, 3169-3179. [ Links ]
17. Sedaghat, T.; Naseh, M.; Khavasi, H. R.; Motamedi, H., Polyhedron 2012, 33, 435-440. [ Links ]
18. Sedaghat,T.; Naseh, M.; Bruno, G.; Amiri Rudbari, H.; Motamedi, H.; J. Mol. Struct. 2012, 1026, 44-50. [ Links ]
19. Sedaghat,T.; Naseh, M.; Bruno, G.; Amiri Rudbari, H.; Motamedi, H.; J. Coord. Chem. 2012, 65, 1712-1723. [ Links ]
20. Azarkish, M.; Sedaghat,T. ; Chin. Chem. Lett. 2012, 23, 1063-1066. [ Links ]
21. Elassar, A. A.; El-Khair, A. A.; Tetrahedron, 2003, 59, 8463-8480. [ Links ]
22. Dey, D. K.; Dey, S. P.; Karan, N. K.; Datta, A.; Lycka, A.; Rosar, G. M.; J. Organomet. Chem., 2009, 694, 2434-2441. [ Links ]
23. Sivasankaran Nair, M.; Joseyphus, R. S.; Inorg. Chim. Acta A, 2008, 70, 749-753. [ Links ]
24. Sari, N.; Gurkan, Perihan; Transit. Metal Chem. 2003, 28, 687-693. [ Links ]
25. Samanta, B.; Chakraborty, J.; Shit, S.; Batten, S. R.; Jensen, P.; Masuda, J. D; Mitra, S.; Inorg. Chim. Acta 2007, 360, 2471-2484. [ Links ]
26. Sonmez, M.; Sekerci, M.; Syn. React. Inorg. Metal-Org. Chem. 2004, 34, 489-502. [ Links ]
27. Singh, R. V.; Fahmi, N.; Biyala, M. K.; J. Iran. Chem. Soc., 2005, 2, 40-46. [ Links ]
28. You, Z. L.; Dai, W. M.; Hu, Y. Q.; Syn. React. Inorg. Metal-Org. Chem. 2008, 38, 451-454. [ Links ]
29. Refat, M. S.; El-Deen, I. M.; Anwer, Z. M.; El-Ghol, S. J.; Mol. Struct. 2009, 920, 149-162. [ Links ]
30. Singh, R. V.; Chaudhary, P.; Chauhan, S.; Swami, M.; Spectrochim. Acta A 2009, 72, 260-268. [ Links ]
31. Angelusiu, M. V.; Barbuceanu, S. F.; Draghici, C.; Almajan, G. L.; Eur. J. Med. Chem. 2010, 45, 2055-2062. [ Links ]
32. Kaczmarek, M. T.; Jastrzab, R.; Kedzia, E. H.; Paryzek, W. R.; Inorg. Chim. Acta 2009, 362, 3127-3133. [ Links ]
33. Singh, R. V.; Chaudhary, P.; Poonia, K.; Chauhan, S.; Spectrochim. Acta A, 2008, 70, 587-594. [ Links ]
34. Mobinikhaledi, A.; Zendehdel, M.; Safari, P.; Hamta, A.; Shariatzadeh, S. M.; Syn. React. Inorg. Metal-Org. Chem. 2012, 42, 165-170. [ Links ]
35. Lobana, T. S.; Sharma, R.; Bawa, G.; Khanna, S.; Coord. Chem. Rev. 2009, 253, 977-1055. [ Links ]
36. Barba, V.;Vega, E.;Luna, R.; Hopfl, H. ; Beltran, H. I.; Zamudio-Rivera, L. S.; J. Organomet. Chem. 2007, 692, 731-739. [ Links ]
37. Farfan, N.; Mancilla, T.; Santillan, R.; Gutierrez, A.; Zamudio-Rivera, L. S.; Beltran, H. I.; J. Organomet. Chem. 2004, 689, 34813491. [ Links ]
38. Rivera, L. S. Z-.; Tellez, R. G-.; Mendoza, G. L-.; Pacheco, A. M-.; Flores, E.; Hopfl, H.; Barba, V.; Fernandez, F. J.; Cabirol, N.; Beltran, H. I.; Inorg. Chem. 2005, 44, 5370-5378. [ Links ]




![One-pot Synthesis of Benzo[c]acridine Derivatives Using SBA-Pr-SO3H as Nano Catalyst](/img/en/prev.gif)









