Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.58 n.2 Ciudad de México Apr./Jun. 2014
Article
Determination of Trace Amount of Lead in Industrial and Municipal Effluent Water Samples Based on Dispersive Liquid-Liquid Extraction
Hamid Shirkhanloo,1 Kaveh Sedighi,2 and Hassan Zavvar Mousavi*,2
1 Iranian Petroleum Industry Health Research Institute (IPIHRI), Occupational and Environmental Health Research Center (OEHRC), Tehran, Iran.
2 Department of Chemistry, College of Science, Semnan University, Semnan, Iran. hzmousavi@semnan.ac.ir
Received August 12th, 2013
Accepted February 10th, 2014
Abstract
In this study, a simple, sensitive and accurate method was developed for the determination of lead ion by combining ionic liquid dispersive liquid-liquid extraction (IL-DLLE) with flame atomic absorption spectrometry. Tetraethylthiuram disulfide (TETD), acetone and 1-octyl-3-methylimidazolium hexafluorophosphate [(C8MIM) (PF6)] were used as the chelating agent, dispersive and extraction solvent, respectively. Under the optimal conditions, the calibration graph was linear in the range of 5-190 μg L-1 of lead and the detection limit was 0.8 μg L-1 with a sample volume of 200 mL. The proposed method was validated by the analysis of one certified reference material and applied successfully to the determination of lead in real water samples.
Key words: Lead; dispersive liquid-liquid extraction; tetraethylthiuram disulfide; flame atomic absorption spectrometry; water samples.
Resumen
En este estudio se desarrolló un método simple, sensible y exacto para la determinación del ion plomo, combinando la extracción dispersiva líquido-líquido iónico (IL-DLLE) y la espectrometría de absorción atómica de flama. Se utilizaron disulfuro de tetraetiltiuram (TETD), acetona y 1-octil-3-metilimidazolium hexafluorofosfato como agente quelante, disolvente de dispersión y disolvente de extracción, respectivamente. Bajo las condiciones óptimas, la gráfica de calibración fue lineal en el intervalo de 5-190 μg L-1 de plomo y el límite de detección fue de 0.8 μg L-1 para un volumen de muestra de 200 mL. El método propuesto fue validado mediante el análisis de un material de referencia certificado y aplicado exitosamente para la determinación de plomo en muestras reales de agua.
Palabras clave: Plomo; extracción dispersiva líquido-líquido; disulfuro de tetraetiltiuram; espectrometría de absorción atómica de flama; muestras de agua.
Introduction
Heavy metals are generally considered to be of sufficient distribution and abundance as to be in some way environmentally or biologically significant as a toxic substance. Heavy metals may originate from various types of sources such as mining, energy and fuel production, fertilizer and pesticide industry, metallurgy, electroplating and atomic energy installation etc. [1-4].
Heavy metals in industrial and municipal effluent water (IMEW) are important sources of water contamination. The contamination and quality of IMEW is the main concern, especially in regions with limited water resources. One of the most important water pollution sources are refinery effluents which can release a large quantity of heavy metals, polycyclic and aromatic hydrocarbons by crude oil-processing and petrochemical industries. In such region, the IMEW samples usually contain high level of heavy metals, which enter into soil, water and plants causing environmental pollutions and poisoning food chain [5-8].
Lead (Pb) is the one of the most hazardous to the human health. Pb (II) cation inhibits biosynthesis and affects the kidneys, brain cells and liver membrane permeability, reducing some of their functions. It can be accumulated in the body and can promote disturbances such as nausea, vomiting, diarrhea, coma and death. Lead pollution in IMEW has influenced the quality of life and may bring serious health problems for both human and animals [8-12]. Therefore, it is necessary to develop sensitive methods for determining lead in IMEW samples [13, 14].
Various techniques such as inductively coupled plasma mass spectrometry [15], luminescence quenching [16], voltammetry [17], co-precipitation [18], flame atomic absorption spectrometry (FAAS) [19], neutron activation analysis (NAA) [20], inductively coupled plasma optical emission spectrometry (ICP-OES) [21] and electro-thermal atomic absorption spectrometry (ET-AAS) [22,23] have been applied for the determination of lead in water samples. Among them, FAAS has been a very attractive technique for routine metal determinations, due to ease of operation, and low acquisition and operating costs compared with others. Determination of lead by FAAS is difficult because of complex formation and significant matrix interferences, which invariably influence normal instrumental analysis. In addition, Pb concentrations in IMEW samples are near or below the limit of detection of FAAS. Therefore, pre-concentration and separation methods such as cloud point [24], microextraction [25], liquid-liquid extraction [26], solid phase extraction [27], single-drop microextraction [28, 29] and dispersive liquid-liquid microextraction (DLLME) [30] can solve the problems above and lead to simplified lead determination by FAAS. Dispersive liquid-liquid microextraction (DLLME) is a miniaturization of the traditional LLE technique, where the extractant phase is a water-immiscible solvent that can be directly immersed in the sample and dispersed by organic solvents or ultrasonic heating. Recently, ionic liquids (ILs) have been used as extraction solvents in DLLME. ILs are organic salts that are liquids at room temperature and have high boiling points. They have various advantages over traditional organic solvents, as well as the possibility of use as extractant phase in water samples [31, 32].
The aim of this work is to combine dispersive liquid-liquid extraction (DLLE) and flame atomic absorption spectrometry for ultra-trace lead determination in IMEW samples. Experimental parameters affecting the extraction process were optimized, and the performance of the proposed method was evaluated.
Experimental
Reagents and chemicals
All reagents were of ultra-trace analytical grade from Merck Company (Darmstadt Germany). Ultrapure water and high purity reagents were used for all preparations of standard and sample solutions. Lead stock solution was prepared from an appropriate amount of the nitrate salt of this analyte as 1000 mg L-1 solution in 0.01 mol L-1 HNO3 (Merck). Standard solutions were prepared daily by dilution of the stock solution. 0.2 mol L-1 acetate buffer solution was used for adjusting pH at 3.2. Polyoxyethyleneoctyl phenyl ether (TX-100) as the anti-sticking agent and tetraethylthiuram disulfide [(C2H5)2NCSS2C SN(C2H5)2)] were purchased from Sigma Aldrich (N:-T24201). Ultrapure water was obtained from the Millipore continental water system (Bedford, USA) and the ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate was obtained from Sigma Aldrich (Germany). All glass vessels used for the trace analysis were kept in 10% nitric acid solution for at least 24 h and subsequently washed with distilled water.
Apparatus
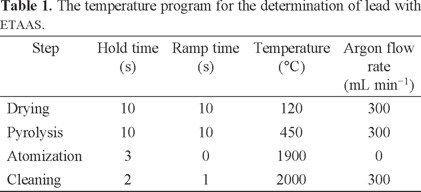
In this study, a flame atomic absorption spectrophotometer (FAAS-932, GBC, Australia) equipped with single-element hollow cathode lamps and air-acetylene burner was used for the determination of Pb (II) ions. All instrumental settings were those recommended in the manufacturer's manual book of GBC. A pH meter, Metrohm E-744 Model (Herisau, Switzerland) equipped with glass electrode was employed for measuring pH values in the water samples. A GBC 932-AUS model GF3000 atomic absorption spectrometer with deuterium background correction and a pyrolytic graphite tube atomizer, equipped with furnace, auto-sampler and a circulating cooling unit, was employed throughout. Measurements were carried out in the peak area mode at 283.3 nm, using a spectral band width of 0.4 nm. The lamp currents were set at 5 mA. The graphite furnace temperature program for the determination of metal ions is given in Table 1.
Sampling
For sampling, all glass tubes were washed with a 0.5 mol L-1 HNO3 solution for at least 24 h and thoroughly rinsed 6 times with ultrapure water before use. As Pb (II) concentration in IMEW samples is very low, even minor contamination at any stage of sampling, sample storage and handling or analysis has the potential to affect the accuracy of results. All IMEW samples were collected from west and east of Tehran refinery (Shahre-ray) and Firoozabad River (Tehran-Varamin).
Procedure
The IL-DLLE preconcentration procedure was performed as follows: first, 5 mL of 2.0 × 10-5 mol L-1 solution of TETD, 0.2 mL of triton X-100 1% (w/v), 5 mL of acetone as a dispersive solvent and a 10 mL buffer solution (pH = 3.2) were added to 200 mL of all standards and samples contained in conical centrifuge tubes, then 0.5 g of IL was added for extraction of Pb (II). Triton X-100, an emulsifier and anti-sticking agent, was added to the solution in order to raise the efficiency of the extraction procedure. For optimizing recovery, 200 mL of 20 μg L-1 Pb (II) standard solution was used instead of the sample, and 0.5 g of 1-octyl-3-methylimidazolium hexafluorophosphate [(C8MIM)(PF6)] was added to the mixed complex. The resulting system was shaken for 2 min by way of ultrasonic shaking at 25 °C. In order to separate the phases, the turbid solution was centrifuged for 4 min at 3500 rpm and the aqueous phase of sample was removed with a transfer pipette before back extraction. Pb (II) was back-extracted from IL twice, by adding 0.5 mL of 1 mol L-1 nitric acid, and the separated aqueous phase was diluted with water up to 1.5 mL. Finally, the aqueous phase was shaken for 1 min and was aspirated into FAAS.
Results and discussion
Effect of pH
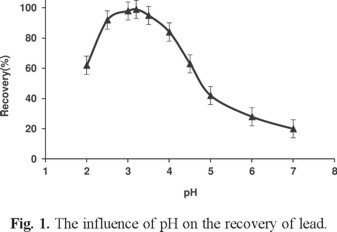
The pH of the sample solution plays an important role in the preconcentration procedure because the formation of soluble metal complexes and their stabilities in aqueous solution are strongly related to the pH of the medium. Optimization of pH was carried out with a set of solutions containing lead metal ions at the concentration given in the general procedure. The pH value of sample solutions was adjusted in a range 1-12 with appropriate buffer solutions. Results showed that the highest extraction efficiency for Pb (II) was achieved at pH 5 to 7. Thus, pH = 6 was selected for further studies (Fig. 1).
Effect of TETD concentration
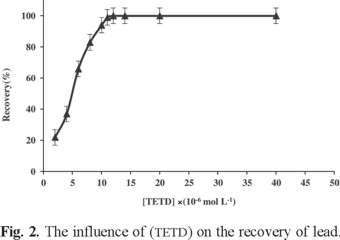
The concentration of tetraethylthiuram disulfide is one of the important parameters for obtaining an optimized DLLE method. The amount of TETD used for these assays was 5 mL of 1 × 10-6 to 1 × 10-4 mol L-1 solutions. The results showed that, by increasing TETD concentration up to 5.0 × 10-5 mol L-1, the recoveries also increased. Fig. 2 shows that 1.5 × 10-5 mol L-1 was the minimum TETD concentration necessary to achieve maximum extraction efficiency, but as we wanted to ensure the whole reaction of trace metal ions with complexing reagent, 2.0 × 10-5 mol L-1 ligand concentration was selected for further studies.
Effect of sample volume and amount of ionic liquid
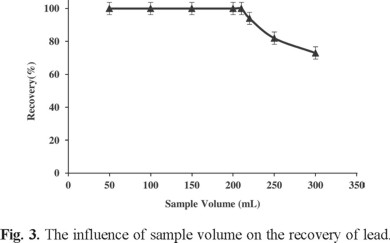
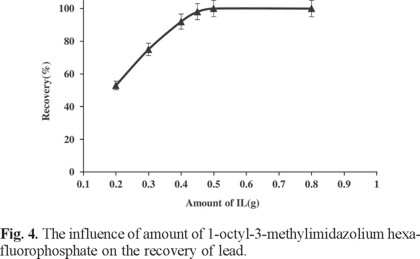
Due to the low concentrations of trace metals in real samples, large sample volumes with the trace metals should be reduced to too much smaller volumes for high preconcentration factor. Hence, the maximum sample volume was optimized by determination of Pb(II) recovery from different sample volumes in the range 50-300 mL. Results are shown in Fig. 3. All recoveries were quantitative until 200 mL and decreased above this volume. In this study, the final solution volume after back extraction was 1.5 mL. In addition, it was observed that extraction efficiency of the system was remarkably affected by ionic liquid amount, so it was examined within the range of 0.2-1 g. Quantitative extraction was observed for IL amount higher than 0.4 g (0.3 mL). Therefore, in order to achieve a suitable pre-concentration, 0.5 g (0.38 mL) of ionic liquid [(C8MIM)(PF6)] was chosen as optimum IL amount, leading to final 0.33 mL of IL recovered at the end of extraction procedure (Fig. 4).
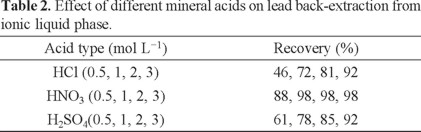
Effect of various mineral acids on the back-extraction
Direct injection of ionic liquids into FAAS was not possible, because ILs have high viscosity. The proposed method was based on back-extraction of lead from IL with a mineral acidic solution. Decreasing of pH leads to complex dissociation and releasing of lead ions into the aqueous phase. Therefore, different mineral acids (HCl, HNO3, H2SO4) were studied for lead back-extraction from the IL phase. The results showed that 1 mol L-1 HNO3 quantitatively extracted lead from the IL phase (Table 2).
Effect of matrix
FAAS is a very specific technique with low sensitivity to interference. Therefore, the potential interference effects occurring in this procedure are mainly related to the extraction process during the preconcentration step applied to target samples. Thus, interferences from coexisting ions should be considered. The effect of potential interferes occurring in environmental water samples on the determination of lead (II) were tested using the optimized conditions of the proposed method. The procedure of DLLE was performed using a 200 ml sample containing 50 μg L-1 of lead in the presence of various amounts of other ions. A contaminant ion was considered to interfere if it resulted in an analytical signal variation of 5%. About 2500-fold excess of Li+, V3+, Mn2+, Ba2+, Cr3+, Cu+2, Zn2+, Cd2+, Ag+, Fe3+, Ni2+ , CH3COO-, Cl-, PO43-, SO42-, F-, NO3-, CO32- could be tolerated. Commonly encountered concomitant ions such as alkali and alkaline earth elements (Na+, Ca2+, Mg2+ and K+) do not form stable complexes with TETD at working pH.
Analytical features
The analytical characteristics of the new method were calculated under the optimized conditions. These characteristics were calculated using the values of the signals for analytical curve. The proposed method showed linearity between 5 and 190 μg L-1 with a determination coefficient (R2) of 0.99. Limit of detection (LOD) was calculated as 3 sb/m, where sb is the standard deviation of the blank and m is slope of the linear section of calibration graph; the LOD value was 0.8 μg L-1. The limit of quantification (LOQ) was 3.0 μg L-1 (obtained for S/N = 10).The enrichment factor of proposed method was 135 and it was calculated by the ratio of angular coefficients of the calibration curves with and without preconcentration [25]. The precision, expressed as a relative standard deviation (R.S.D, n = 10), was 2.8% for lead solution containing 50 ng mL-1 and 3.2% for a lead solution having 120 ng mL-1.
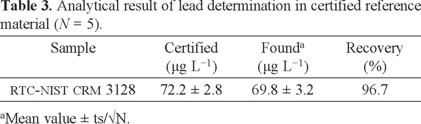
Method validation
The validation of the presented procedure was performed by analysis of a certified reference material for lead values (RTC-NIST CRM 3128). The certified and observed values for certified reference material are provided in Table 3. The results found were in agreement with the certified values of CRM. Also, validation of the methodology was confirmed by using directly analyzed lead in water samples with Mg (NO3)2 modifier and electro-thermal atomic absorption spectrometry (ETAAS), results are given in Table 4.
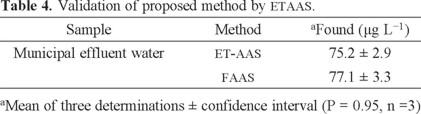
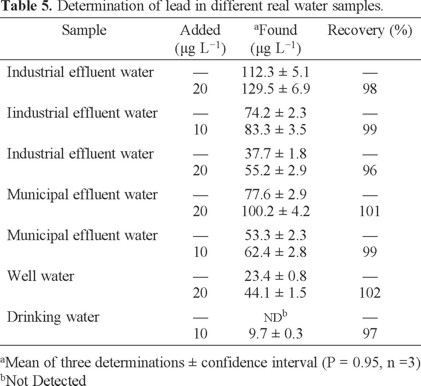
Since it was found that the proposed method was very useful for the preconcentration of ultra-trace lead in the presence of interferences, the method was applied to the analysis of industrial effluent water (IEW), drinking water (DW), well water (WW) and municipal effluent water (MEW) samples under optimal experimental conditions. The obtained results are presented in Table 5, and the recoveries varied in the range 96-102%. The results showed that the proposed method is comparable with respect to other powerful techniques as ET-AAS and ICP (Table 6).
Conclusions
In this research, IL-DLLE combined with FAAS was used to develop a new procedure for the determination of trace amounts of Pb (II) in water samples. The developed method is simple, rapid, selective and sensitive and can be used for trace lead determination in environmental samples. The enrichment factor was 135. A limit of detection (LOD) of 0.8 μg L-1 was achieved at pH 3.2 with a sample volume of 200 mL. Validation of the methodology was confirmed using certified reference material (CRM) and ET-AAS. TETD has difference with sodium diethyl dithiocarbamate (NaDDC), dithizone and ammonium pyrroldin edithiocarbamate (APDC), used as chelating agents in many references. TEDT faster transported Pb into IL and the efficiency of extraction was superior to others. Also, TETD is immiscible in water and removal from water at the end of extraction is easy. The results show that the method is comparable with respect to other powerful techniques such as ET-AAS and ICP. The proposed method was successfully applied to the determination of Pb (II) at low levels in different water samples.
Acknowledgments
The authors are thankful to Iranian Petroleum Industry Health Research Institute (IPIHRI) and Research Institute of Petroleum Industry (RIPI) for their support for this work.
References
1. Heidari, A.; Younesi, H.; Mehraban, Z. Chem. Eng. J. 2009, 153, 70-79. [ Links ]
2. Ahmaruzzaman, M. Adv. Colloid Interfac. 2011, 166, 36-59. [ Links ]
3. Wang, J. A.; Chen, C. Biotechnol.Adv. 2006, 24, 427-451. [ Links ]
4. Jalali, R.; Ghafourian, H.; Asef, Y.; Davarpanah, S. J.; Sepehr, S. J. Hazard. Mater. 2002, 92, 253-262. [ Links ]
5. Okoronkwo, N. E.; Igwe, G. C.; Onwuchekwa, E. C. African J. Biotechnol. 2005, 4, 1521-1524. [ Links ]
6. Dhankhar, R.; Chhikara, S.; Rana, L.; Sangwan, S. Eur. J. Soil Biol. 2009, 45, 459-465. [ Links ]
7. Atubi, O. Am. Rev. Pol. Econ. 2011, 9, 45-56. [ Links ]
8. Asgharipour, M. R.; Sirousmehr, A. R. Ann. Biol. Res. 2012, 3, 1094-1101. [ Links ]
9. US Department of Health & Human Services, Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Lead, Atlanta, 2007. [ Links ]
10. Chober, S. E.; Mirel, L. B.; Graubard, B. I. Environ. Health Perspect. 2006, 114, 1538-1541. [ Links ]
11. U.S. Environmental Protection Agency, Environmental Criteria and Assessment Office.; Lead exposures in the human environment, EPA600D86185, 1985. [ Links ]
12. Vilar, M.; Barciela, J.; Garcia-Martin, S.; Pena, R. M.; Herrero, C. Talanta 2007, 71, 1629-1636. [ Links ]
13. World Health Organization (WHO). Guidelines for Drinking Water Quality; Second addendum to 3rd Ed, 2008. [ Links ]
14. U.S. Environmental Protection Agency (EPA), Drinking Water Standards and Health Advisories; EPA 822-R-09-011, Washington, 2009. [ Links ]
15. Christopher, D. J.; Kamal, S. Isotopes Environ. Health Stud. 2010, 46, 484-494. [ Links ]
16. Yang, X.; Zhu, Y.; Liu, P.; He, L.; Li, Q.; Wang, Q.; Wang, K.; Huang, J.; Liu, J. Anal. Methods 2012, 4, 895-901. [ Links ]
17. Ijeri, V. S.; Srivastava, A. K. Anal. Sci. 2001, 17, 605-608. [ Links ]
18. Citaka, D.; Tuzena, M.; Soylakb, M. Food Chem. Toxicol. 2009, 47, 2302-2307. [ Links ]
19. Mortazavi, K.; Ghaedi, M.; Roosta, M.; Montazerozohori, M. Indian J. Sci. Technol. 2012, 5, 1893-1900. [ Links ]
20. Faanu, A.; Ephraim, J. H.; Darko, E. O.; Kpeglo, D. O.; Lawluvi, H.; Adukpo, O. Res. J. Environ. Earth Sci. 2011, 3, 178-187. [ Links ]
21. Depoi, F. S.; Oliveira, T. C.; Moraes, D. P.; Pozebon, D. Anal. Methods 2012, 4, 89-95. [ Links ]
22. Mizuguchi, H.; Ishida, M.; Takahashi, T.; Sasaki, A. Anal. Sci. 2011, 27, 85-89. [ Links ]
23. Mohammadi, S. Z.; Shamsipur, T.; Karimi, M. A.; Naroui, E. The Sci. World J. 2012, Article ID 640437, 6 pages doi:10.1100/2012/640437. [ Links ]
24. Shah, F.; Kazi, T. G.; Afridi. H. I.; Arain, M. B.; Baig, J. A. J. Hazard. Mater. 2011, 192, 1132-1139. [ Links ]
25. Wang, Y.; Ke, X.; Zhang, J.; Du, X.; Ma, J.; Li, J. Bull. Chem. Soc. Ethiop. 2012, 26, 9-18. [ Links ]
26. Bouabdallah, I.; Zidane, I.; Hacht, B.; Touzani, R.; Ramdani, A. Arkivoc J. 2006, Part (xi), 59-65. [ Links ]
27. Moghimi, A. Aust. J. Basic & Appl. Sci. 2012, 6, 320-330. [ Links ]
28. Nazari, S. Trade Sci. Inc. 2012, 11, 128-132. [ Links ]
29. Cheng, K.; Choi, K.; Kim, J.; Sung, H.; Chung, D. S. Microchem. J. 2013, 106, 220-229. [ Links ]
30. Gharehbaghi, M.; Shemirani, F. Clean Soil- Air-Water, 2012, 40, 290-297. [ Links ]
31. Zhang, D.; Wang, W.; Deng, Y.; Zhang, He Zhao, J.; Chen, J. Chem. Eng. J. 2012, 179, 19-25 [ Links ]
32. Zhang, L.; Chen, J.; Jin, W.; Deng, Y.; Tian, J.; Zhang, Y. J. Rare Earth, 2013, 31, 1195-1201. [ Links ]
33. Antonio, V. H. H.; Javier, H. B.; Teresa, M. B. M.; Miguel, A. R. D. J. Electrophoresis 2010, 31, 3457-3465. [ Links ]
34. Silva, E. L.; Roldan, P. S. J. Hazard. Mater. 2009, 161, 142-147. [ Links ]
35. Doner, G.; Ege, A. Anal. Chim. Acta 2005, 547, 14-17. [ Links ]
36. Matoso, E.; Kubota, L.T.; Cadore, S. Talanta 2003, 60, 1105-1111. [ Links ]
37. Chen, J.; Teo, K. C. Anal. Chim. Acta 2001, 450, 215-222. [ Links ]
38. Li, Z.; Tang, J.; Pan, J. Food Control 2004, 15, 565-572. [ Links ]
39. Manzoori, J. L.; Amjadi, M.; Abulhassani, J. Anal. Chim. Acta 2009, 644, 48-59. [ Links ]
40. Bai, H.; Zhoua, Q.; Xie, G.; Xiao, J. Talanta 2010, 80, 1638-1642. [ Links ]
41. Haixia, S.; Zaijun, L.; Ming, L. Microchim. Acta 2007, 159, 95-100. [ Links ]
42. Abulhassani, J.; Manzoori, J. L.; Amjadi, M. J. Hazard. Mater. 2010, 176, 481-486. [ Links ]














