Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.58 n.2 Ciudad de México Apr./Jun. 2014
Article
Polyethylene-Waste Tire Dust Composites Via In Situ Polymerization
Yadira Karina Reyes Acosta,1 Rosa Idalia Narro Céspedes,1 María Guadalupe Neira Velázquez,2 José Díaz Elizondo,2 Francisco Enríquez-Medrano,2 Luis Alexandro Valencia López,2 María Elena Ramos Aguiñaga,1 Hened Saade Caballero,2 and Ramón Díaz de León2*
1 Facultad de Ciencias Químicas, Universidad Autónoma de Coahuila, Blvd. V. Carranza, s/n, Saltillo, Coahuila, México. CP 25280.
2 Centro de Investigación en Química Aplicada (CIQA), Blvd. Enrique Reyna Hermosillo, No. 140, Col. San José de los Cerritos, Saltillo, Coahuila, México. CP 2529325294. ramon.diazdeleon@ciqa.edu.mx
Received November 7th, 2013
Accepted January 28th, 2014
Abstract
Polyethylene/waste tire dust (WTD) composites were obtained by an in situ polymerization technique. The surface of the WTD was modified with deposition of polyethylene by using plasma polymerization. Ethylene polymerization was carried out using bis(cyclopentadienyl) titanium dichloride (Cp2TiCl2) as homogeneous metallocene catalyst, while diethylaluminum chloride (DEAC), ethylaluminum sesquichloride (EASC) and methyl aluminoxane (MAO) were used as co-catalysts at two different [Al]/[Ti] molar ratio. The main characteristics of the obtained polyethylenes were determined by size exclusion chromatography, thermogravimetric analysis, differential scanning calorimetry and wide-angle X-ray diffraction. The results showed that by using EASC and MAO the highest catalytic activities were presented at a [Al]/[Ti] molar ratio of 9.17 and 18.33 respectively. Even though it was possible to obtain polyethylene using WTD (modified or unmodified) the catalytic activity was lower than in the case in which no WTD was added in ethylene polymerization. Scanning transmission electronic microscopy images evidenced that the original morphology of the polyethylenes was not modified by the presence of WTD.
Key words: Polyethylene, waste tire dust, metallocene catalyst.
Resumen
Fueron obtenidos compositos de polietileno/residuo de polvo de llanta (WTD) mediante la técnica de polimerización in situ. La superficie del WTD fue modificada mediante polimerización por plasma de etileno. La polimerización de etileno se llevó a cabo utilizando un catalizador del tipo metaloceno dicloro-di-ciclopentadienil titanio (IV) (Cp2TiCl2), empleando diferentes co-catalizadores tales como cloruro de dietil aluminio (DEAC), sesquicloruro de etil aluminio (EASC) y metil aluminoxano (MAO). En las polimerizaciones de etileno se emplearon dos diferentes relaciones molares de [Al]/[Ti]. Las principales características de los polietilenos obtenidos fueron determinadas mediante cromatografía por exclusión por tamaños, análisis termogravimétrico, calorimetría diferencial de barrido, difracción de rayos X y microscopía electrónica de barrido. Los resultados obtenidos muestran que el EASC y MAO presentan la mayor actividad catalítica a relaciones [Al]/[Ti] de 9.17 y 18.33, respectivamente. Aunque es posible polimerizar etileno en presencia de WTD y obtener los compositos, la presencia de WTD modificado y sin modificar disminuye la actividad catalítica. Por su parte, mediante microscopía electrónica de barrido se pudo evidenciar que la morfología de los polietilenos obtenidos no se modifica por la presencia del WTD.
Palabras clave: Polietileno, polvo de llanta reutilizado, catalizador metaloceno.
Introduction
Discarded tires represent an important contribution of non-degradable materials on waste deposits, thus recyclability of these rubbers is environmentally necessary and economically attractive, however the matrix phase in tires is a vulcanized rubber that cannot be dissolved or molten, limiting their recyclability when it is expected to be used as powdered tires, most commonly known as waste tire dust (WTD). WTD has been used as filler in thermoplastics, thermosets or virgin rubbers [1-5]. Polyethylene (PE) [6-9] and polypropylene (PP) [10-12] are thermoplastics widely reported to prepare WTD filled compounds, although some others such as ethylene-vinyl-acetate (EVA) [13, 14] and polystyrene [15] have been also reported.
PP has been the most studied thermoplastic in this topic. For example, Egodage et al. [16] reported the preparation of waste-PP/WTD blends by melt-mixing from 10 to 90 wt-% of PP. From optical micrographs they observed WTD like the dispersed phase in blends containing maximum 60 wt-% of WTD, nonetheless, at a higher percentage the phase was inverted and the dispersed phase was now corresponded to the PP. A maximum tensile strength of 18.6 MPa was obtained with the 90 wt-% of WTD and the maximum elongation at break was obtained with the 10 wt-% of WTD (27.5%).
On the other hand, the use of compatibilisers agents or the surface modification of WTD with techniques like UV radiation or acid treatment are utilized to have a better affinity between the thermoplastic and the filler, ensuring an effective integration of WTD with the thermoplastic matrix. For example, Hrdlicka et al. [17] reported the preparation of low-density-PE/WTD composites via compounding of molten PE and WTD in an internal mixer. PE composition was 50 wt-% and some compatibilisers (based on dicumyl peroxide or sulfur) were added in order to improve its dispersion as well as the mechanical properties of the resulting thermoplastic elastomers with a tensile strength around 8.0 MPa and an elongation at break close to 100%. PE/WTD/EPDM composites were also prepared and analyzed with different PE compositions. Hyo Lee et al. [18] reported the surface modification of WTD with UV treatment in presence of allylamine. The degree of grafting on the WTD was confirmed by FTIR, TGA and other kind of analyses. The PP/WTD composites were prepared by blending in a twin screw extruder. The mechanical properties of the composites were influenced by several factors like the UV radiation time, WTD loading and allylamine concentrations. In a general way the mechanical properties of modified WTD composites were better than unmodified WTD composites.
As it can be observed all previous works related with the production of WTD filled compounds utilize a mechanical mixing technique, however exists another much less-studied option to prepare this kind of materials that offers a better alternative to achieve a good dispersion of the WTD in the thermoplastic component. It is known as the in situ polymerization technique and consists on the dispersion of the filler in the monomer or solvent/monomer mixture and then polymerize (by radical, anionic or coordination methods) to produce the corresponding polymer containing the well-dispersed filler on it.
Here we report the use of the in situ polymerization technique to prepare PE/WTD composites. WTD was modified by an ethylene plasma treatment to confer a better affinity between the PE continuous phase and the WTD dispersed phase. Unmodified WTD was also studied for comparing results. The bis(cyclopentadienyl) titanium dichloride (Cp2TiCl2) was the catalyst utilized to polymerize the ethylene monomer. This work is focused on preparation of composites by this novel technique and analysis of the catalytic activity in polymerizations, the molecular weight of the synthesized PE, as well as the morphology and thermal properties of composites.
Experimental part
Reactants
All manipulations were carried out under inert atmosphere using a dual vacuum-argon line and standard Schlenk techniques or in a glove box. Hexane was distilled from sodium under argon before use. As catalyst the bis(cyclopentadienyl) titanium dichloride (Cp2TiCl2) was used and as co-catalysts diethylaluminum chloride (DEAC 1 M), ethylaluminum sesquichloride (EASC 1 M) and methyl aluminoxane (MAO 10 wt-% in toluene) were used. All previous chemicals were purchased from Aldrich and were used as received. Polymerization-grade ethylene was purchased from Praxair and it was purified by passing it through 4 Å activated molecular sieves. WTD with an average particle diameter of 0.125 mm was purchased from Genbruger.
Surface modification of WTD by ethylene plasma
The modification was carried out in a plasma reactor, which consists of a glass flask surrounded by a copper wire that is connected to a radio frequency generator (RFG). Details of this reactor are illustrated on reference [19]. Plasma treatment began with the introduction of 0.5 g of WTD into the glass flask, followed by air evacuation. Then, the ethylene monomer flow was fixed by maintaining a constant pressure of 2.5 × 10-5 bar in the supply tank, while the RFG was operated at 50 W for 60 min. The modified WTD (WTD-M) was analyzed by TGA.
Ethylene polymerization
All polymerizations were performed in a 2 L titanium Parr reactor through the following procedure. The WTD was added to the reactor and several vacuum-argon cycles at 100 °C were carried out. The reactor was cooled to room temperature and filled with 300 mL of distilled hexane under argon atmosphere. The reactor was heated to reach the established temperature under 100 rpm of agitation. The catalyst system was fed into the reactor using a syringe. The polymerization was started by adding the monomer at a continuous flow. All experiments were performed at 50 °C and under ethylene pressure of 5 bar in the supply cylinder by 1 hour. The reactions were finished by releasing the monomer pressure. The obtained PE was precipitated in acidified methanol and dried under vacuum until constant weight. Both, titanocene catalyst and alkylaluminum co-catalyst concentrations were chosen for an easy temperature control and to obtain enough measurable amounts of PE.
Characterization
Size exclusion chromatography (SEC). The molecular weight distributions were determined with an experimental error of approximately 5% by SEC using an Alliance chromatograph (GPCV-2000) equipped with two on-line detectors: a differential viscometer and a differential refractometer. Calibration was carried out with polystyrene standards and 1,2,4-trichlorobenzene was used as eluent. Measurements were carried out at a flow rate of 1 mL/min and 140 °C.
Differential scanning calorimeter (DSC). Thermograms were obtained using an instrument DSC 2920 at a heating rate of 10 °C/min under inert atmosphere. Each sample was heated twice in order to eliminate thermal history. The melting temperature (Tm) was taken as the peak temperature of the endothermic process. Crystallinity (Xc) values were determined taking the ΔH value provided by the DSC analysis and the value of 293 J/g for the fully crystalline PE (ΔH0) [20] and using the following equation:
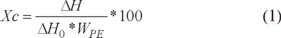
Where WPE is the weight fraction of PE in the case of composites.
Thermogravimetric analysis (TGA). The analyses were performed with an experimental error of approximately 3% using a TA-Q500 apparatus in which the samples were heated at 10 °C/min, in air or a nitrogen atmosphere, under a flow rate of 50 mL/min. Curves were recorded from 50 to 650 °C.
Wide-angle X-ray diffraction (WAXD). X-ray measurements were carried out in a Siemens D5000 diffractometer with a Ni-Filtered CuKα radiation generator (wave length of 1.5406 Å). Patterns were recorded from 5 to 40° (in 2θ) with 5 s standing per step.
Scanning electron microscopy (SEM). Morphology was determined by using a JEOL JSM-7401 F microscope with a secondary electrons detector (SEI).
Results and Discussion
WTD composition was obtained through TGA, containing 56.4 wt-% of rubber, 8.6% of volatile products, 30.5% of carbon black and 4.5% of ashes. Surface modification of WTD was verified by TGA. Fig. 1 shows the thermograms of WTD unmodified (WTD-U) and modified (WTD-M). The weight loss was very similar for both materials in the first zone (up to 350 °C) with approximately 14 wt-%, which mainly corresponds to volatile compounds such as plastifiers, oils and antioxidants. In the second zone, between 350 and 500 °C, a very prominent loss of approximately 52 wt-% was observed, which involves elastomers, curing agents and processing agents. Finally over 600 °C, a loss of the remaining 34 wt-% of WTD was observed. This loss was attributed to carbon black and Zn containing compounds. Fig. 1b shows closer the first zone of thermograms and it can be observed a difference of approximately 2 wt-% in the weight loss of both materials. It is clear that WTD-M with ethylene plasma presents a higher thermal stability, which is attributed to the surface barrier of highly crosslinked PE. Similar improvement in thermal stability provided by the ethylene plasma has been observed in other substrates such as carbon fiber [21].
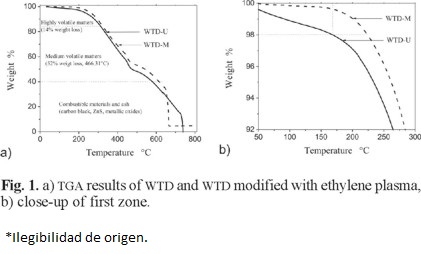
Table 1 shows the results of catalytic activity and molecular weights obtained in the different polymerizations. The selected [Al]/[Ti] ratios led to catalytic activities ranging between 1.25-34.99 in those runs in which WTD was not included (runs 1-6). In the cases of DEAC and EASC as co-catalysts lower catalytic activities were observed at higher [Al]/[Ti] ratios; however, even when this unexpected effect has been previously reported [22], a satisfactory explanation has not been provided.
In the case of MAO as co-catalyst, the catalytic activity was considerably improved at a higher ratio [Al]/[Ti] as result of the formation of more active centers. This behavior observed with MAO was expected since it has been reported as an efficient activator for metallocene catalysts for olefin polymerization [23, 24]. In addition to this, MAO is the responsible of alkylating halogenated metallocene complexes in which initially a monomethyl compound is rapidly formed and subsequently the excess of MAO leads to the dialkylated species. It has been reported that at least one alkyl group has to be bonded to the metallocene to be able of being activated
In both [Al]/[Ti] utilized ratios, DEAC was the co-catalyst that originated the higher molecular weights, which suggests that by employing this compound as co-catalyst, less chain transfer reactions were presented, since It is well known that the molecular weight of polyethylene chains fundamentally depends on chain transfer reactions which lead to the termination of the growth of the polymer chains, being the aluminum transfer reaction the most presented, as well as the β-elimination. Another aspect that influence on the molecular weight of the polymer chains is the propagation rate, as it can be appreciated from the following equation for the polymer chain length (Xn) [25]:
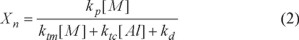
Where kp is the constant rate for the propagation reaction, [M] is the monomer concentration, [Al] is the co-catalyst concentration, ktm and ktc are the chain-transfer rate constants to monomer and co-catalyst respectively, and kd is the constant rate for catalyst deactivation. The propagation rate is influenced by several factors, such as the reaction conditions, monomer pressure and the coordination wielded by the co-catalyst which can prevent the monomer coordination and in this way decrease the propagation rate. Thus a higher propagation rate from DEAC and EASC would be expected; however it was not determined in this work.
Data in Table 1 and Fig. 2 indicate that in the cases of DEAC and MAO the polydispersity (PDI) diminishes as the concentration of co-catalysts increases. The molecular weight distributions (MWD) of the synthesized PE in the presence of EASC showed two well-defined populations in both [Al]/[EASC] utilized ratios (see Fig. 2b). This behavior can be explained by the existence of more than one type of active sites performing the polymerization [26]. On the other hand, the asymmetry of the Gauss functions of MAO and DEAC, besides of their high PDI values, induces that, as well as EASC, these compounds leads to multimodality, i.e. present multiple active sites carrying out the polymerization. It has been previously reported that the multimodal gauss graphs obtained by using MAO (see Fig. 3) as activator are generated by the presence of residual TMA, which is the synthesis predecessor of MAO; however the reason of the multimodality of EASC and DEAC has not been reported.
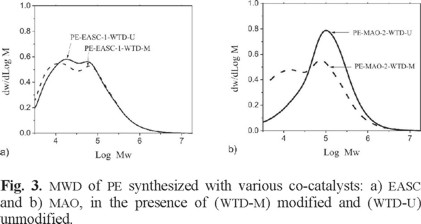
DEAC was not evaluated in the ethylene polymerizations in presence of WTD because the poor catalytic activity presented in runs 2 and 5. For ethylene polymerizations in the presence of WTD, [Al]/[Ti] ratios of 9.17 and 18.33 were utilized for EASC and MAO respectively, since these ratios were the best in catalytic activity for each co-catalyst (run 1 and 6, respectively). Results of catalytic activity and molecular weight for these runs are presented also in Table 1. For both kinds of WTD (modified or unmodified), a decrease in the catalytic activity and in the molecular weight was observed, as well as an increase in the PDI values. These results suggest that, since the purity requirement of these catalyst systems is very high, It is believed that the impurities contained by WTD, such as black carbon and volatile products, as well as possible functional groups, deactivated the catalyst system. Therefore, the vacant coordination positions in possible active centers become unavailable for monomer complexation and subsequent ethylene polymerization. The bimodal distribution obtained when WTD-M was used (see Fig. 3), can be attributed to the participation of more than one active species as well as to the reactivation process typical of MAO.
Fig. 4 shows the micrographs of the different PE obtained in absence of WTD. It is shown that the PE obtained in a [Al]/[Ti] ratio of 9.17 with EASC (run 1, Fig. 4 a) and DEAC (run 2, Fig. 4 b) presented morphologies similar to coral or rose in contrast to the obtained when MAO was used (run 3, Fig. 4 c) that shows small globular agglomerates with spider web. When the [Al]/[Ti] ratio was increased to 18.33, the morphology of the PE obtained with EASC remains unchanged (run 4, Fig. 4 d). However, the polymer prepared with DEAC (run 5, Fig. 4 e) changed to small agglomerates with disperse worm-like and spider web forms, while the morphology of the PE obtained with MAO (run 6, Fig. 4 f) changed to a mixture of small and large globular agglomerates with spider web forms.
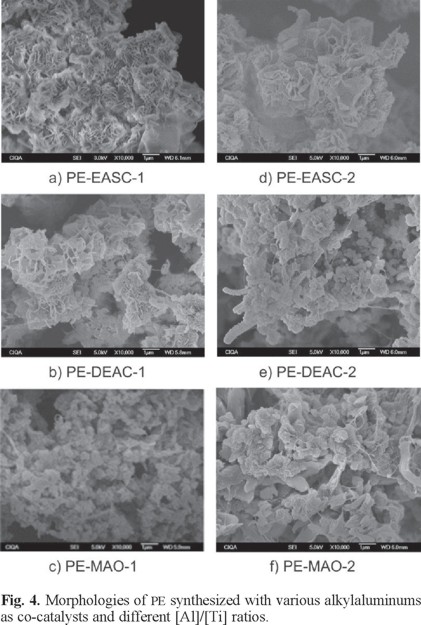
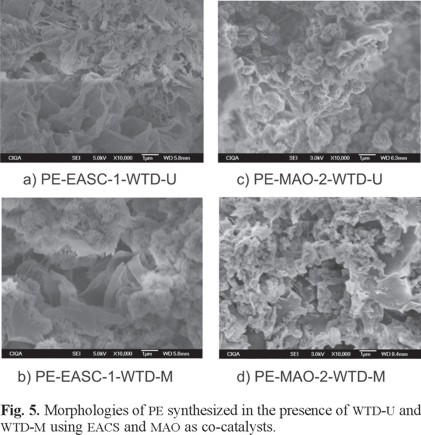
Fig. 5 shows the micrographs of the different PE obtained in presence of WTD (modified and unmodified). For the [Al]/[Ti] ratio of 9.17 when EASC was used as co-catalyst and WTD-U and WTD-M were incorporated (runs 7 and 8), morphologies of coral or rose and laminated type were obtained (Fig. 5 a and 5 b). When WTD-U and WTD-M were added in a [Al]/[Ti] ratio of 18.33 using MAO (runs 9 and 10), the obtained morphologies were small globular agglomerates with disperse laminar shapes (Fig. 5 c and 5 d), similar to those obtained in absence of WTD. These results indicate that the presence of WTD in the synthesis of PE does not produce significant variations on the final morphology. Even when the exact reason of the formation of these morphologies remains unknown, it is suggested that the formation of the different surface morphologies depends fundamentally on the way in which the polymer chain grow beginning from the active centres. The factors that could influence on these morphologies are the crystallinity degree and the chain branching density.
The X-ray diffraction patterns (XRDP) of PE obtained with EACS and MAO in presence of WTD (modified and unmodified) are shown in Fig. 6. For comparative purposes, XRDP of the obtained PE when WTD was not used was also included. All of them show the main signals of the typical crystalline structure of high density PE. The signals at 21.6 and 23.8 2θ °Correspond to the [110] and [200] planes respectively, which are characteristic of a unitary orthorhombic cell of PE [27]. The amorphous halo at approximately 19 2θ° is negligible, indicating high crystallinity [28]. The presence of WTD (unmodified and modified) during the ethylene polymerization does not change the original crystal structure.
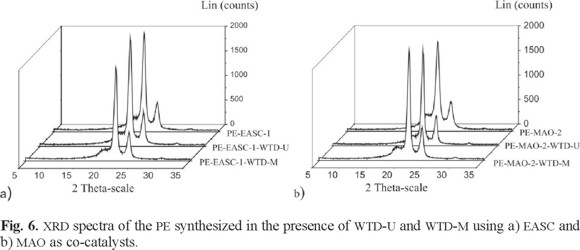
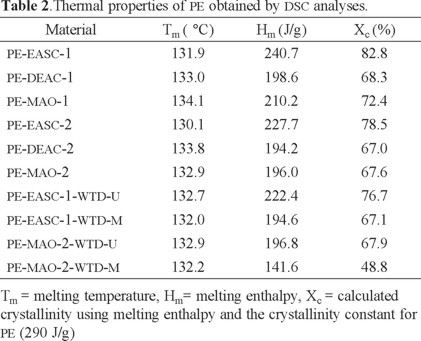
Fig. 7 shows the DSC analyses of the PE synthesized in presence of WTD, while the Table 2 shows the DSC results for all PE reported in Table 1. The obtained PE presented a Tm value between 130 and 134 °C, with or without WTD. EASC lead to obtain the highest proportion of crystalline phase, according to the Xc values, at low and high [Al]/[Ti] ratios. It was observed that the presence of WTD during the polymerization reduced the crystallinity of the resulting PE. To explain the observed behaviors it has to be considered that the PE melting process is mainly related to the short chain branching density, since by increasing short chain branching density the lamellar thickness of the crystal structure is decreased as well as the cristallinity degree [29]. In addition, the crystallinity can be reduced by incorporating fillers that hinder the crystalline alignment of polymer chains [30]. Given the above information, it is considered that EASC produces PE with a lower level of short chain branching. On the other hand, the presence of WTD during the ethylene polymerization would favor the formation of short chain branching and/or hinder the alignment of the polymer chains.
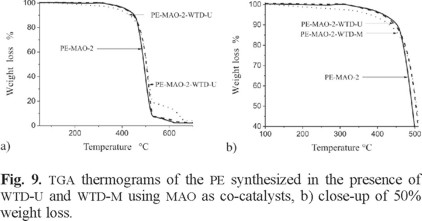
The TGA determinations of the synthesized PE in presence of WTD are shown in Fig. 8 and 9. A weight loss was registered in all cases in the range of 250-510 °C. A similar weight loss pattern was reported by Abdul et al. [30] and Reza et al. [31] for high density PE.
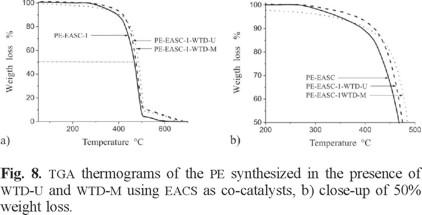
In Fig. 8, the main corresponding weight loss temperature values for PE prepared using EASC ([Al]/[Ti] = 9.17) in absence of WTD, as well as with WTD-U and WTD-M, were 467, 475 and 485 °C respectively. PE/WTD-M composite shows a slightly better thermal stability than PE/WTD-U composite. This behavior can be attributed to the interactions between the polyethylene matrix and the polyethylene deposited in WTD-M, also plasma modified WTD possess better thermal stability than unmodified WTD (see Fig 1).
In accordance with the data in Fig. 9, the main corresponding weight loss temperature values for PE prepared with MAO ([Al]/[Ti] = 18.33) was 492 °C in absence of WTD and the same value of 502 °C for the PE/WTD-U and PE/WTD-M composites. These results indicate that the thermal stability of the PE/WTD-M composites is higher than the other composites tested, regardless whether this is modified or unmodified. It is to be noticed that nanocomposites PE/doped-TiO2 [30] and PS/ organoclay [32] obtained via in situ polymerization, also showed a higher thermal stability than the pure polymer.
Conclusions
The catalytic polymerization of ethylene using metallocenes in the presence of WTD allows to obtain composites containing high density polyethylene (HDPE). The higher catalytic activity was obtained using a [Al]/[Ti] ratio of 18.33 and MAO as co-catalyst, in the presence or absence of WTD. Also the highest thermal stability was obtained when MAO was used. Using WTD in the process a decrease in the catalytic activity during the polymerization and in the PE crystallinity was shown, however, composites with enhanced thermal stability were obtained. The findings reported here could be the basis for a future development of a process for the production of PE/WTD composites via in situ polymerization.
Acknowledgments
The authors thank to CONACYT for the financial support through the project 105388, as well as Ricardo Mendoza, Jesús Cepeda, Uriel Peña, Judith Cabello and Francisco Zendejo for their technical support.
References
1. Yasin, T.; Khan, S.; Nho, Y. C.; Ahmad, R. Rad. Phys. Chem. 2012, 81, 421-425. [ Links ]
2. Aman, A.; Yaacob, M. M.; Abd Razak, J. Aust. J. Basic Appl. Sci. 2011, 5, 1578-1583. [ Links ]
3. Ismail, H.; Omar, N. F.; Othman, N. BioResources 2011, 6, 3742-3756. [ Links ]
4. Ismail, H.; Omar, N. F.; Othman, N. J. Appl. Polym. Sci. 2011, 121, 1143-1150. [ Links ]
5. Karger-Kocsis, J.; Meszaros, L.; Barany, T. J. Mat. Sci. 2013, 48, 1-38. [ Links ]
6. Zhu, J.; Zhang, X.; Liang M.; Lu, C. J. Polym. Res. 2011, 18, 533-539. [ Links ]
7. Sonnier, R.; Leroy, E.; Clerc, L.; Bergeret, A.; Lopez-Cuesta, J. M. Polym. Testing 2007, 26, 274-281. [ Links ]
8. Tolstov, A.; Grigoreyeva, O.; Fainleib, A.; Danilenko, I.; Spanoudaki, A.; Pissis P.; Grener, J. Macromolecular Symposia 2007, 254, 226-232. [ Links ]
9. Punnarak, P.; Tantayanon S.; Tangpasuthadol, V. Polym. Degrad. Stab. 2006, 91, 3456-3462. [ Links ]
10. Ismail, H.; Awang, M.; Hazizan, M. A. Polym. Plast. Technol. Eng. 2006, 45, 463-468. [ Links ]
11. Ismail, H.; Awang, M. J. Polym. Environm. 2008, 16, 147-153. [ Links ]
12. Zainal Z.; Ismail, H. J. Vinyl Addit. Technol. 2011, 17, 245-253. [ Links ]
13. Ramli, S.; Ratnam, C. T.; Ahmad, S. H. Malaysian Polym. J. 2013, 8, 27-32. [ Links ]
14. Anis Sakinah, A. A.; Ratnam, C. T.; Luqman Chuah, A.; Yaw, T. C. S. J. Elastom. Plast. 2011, 43, 239-256. [ Links ]
15. Zhang, J.; Chen, H.; Zhou, Y.; Ke C.; Lu, H. Polym. Bull. 2013, 70, 2829-2841. [ Links ]
16. Egodage, S. M.; Harper J. F.; Walpalage, S. J. Nat. Sci. Found. Sri Lanka 2009, 37, 117-123. [ Links ]
17. Hrdlicka, Z.; Kuta A.; Hajek, J. Polimery 2010, 55, 832-838. [ Links ]
18. Lee, S. H.; Zhang, Z. X.; Xu, D.; Chung, D.; Oh, G. H.; Kim, J. K. Polym. Eng. Sci. 2009, 49, 168-176. [ Links ]
19. Neira Velázquez, M. G.; Ramos de Valle, L. F.; Hernández Hernández, E.; Ponce Pedraza, A.; Solís Rosales, S. G.; Sánchez Valdez, S.; Bartolo Pérez, P.; González González, V. A. Plasma Proc. Polym. 2011, 8, 842-849. [ Links ]
20. D'Amato, M.; Dorigato, A.; Fambri L.; Pegoretti, A. eXPRESS Polym. Lett. 2012, 6, 954-964. [ Links ]
21. Hernández Hernández, E. (2010) PhD Thesis, Centro de Investigación en Química Aplicada, Saltillo, Coahuila, México. [ Links ]
22. Xian Chen, E. Y.; Marks, T. J. Chem. Rev. 2000, 100, 1391-1434. [ Links ]
23. Lena, F.; Chen, P.; Helv. Chim. Acta 2009, 92, 890-896. [ Links ]
24. Zohuri, G.; Jamjah, R.; Ahmadjo, S. Iran. Polym. J. 2005, 14, 111-116. [ Links ]
25. Ahmadi, M.; Jamjah, R.; Nekoomanesh, M.; Zohuri, G.; Arabi, H. Iran. Polym. J. 2007, 16, 133-140. [ Links ]
26. Jian, S.; Wang, L.; Zhang, P.; Feng, L. Macromol. Theory Simul. 2002, 11, 77-83. [ Links ]
27. Ren, X.; Wang, X. Q.; Zhong, G.; Fuqua M. A.; Ulven, K. J. Appl. Polym. Sci. 2008, 107, 2837-2845. [ Links ]
28. Ye, Z.; Zhu, S.; Wang, W.; Alsyouri A.; Lin, Y. S. J. Polym. Sci. Part B: Polym. Phys. 2003, 41, 2433-2443. [ Links ]
29. Alobaidi, F.; Ye Z.; Zhu, S. Polymer 2004, 45, 6823-6829. [ Links ]
30. Abdul, S. H.; Kottukkal, B.; Masihullah J.; Al-Harthi, J. M. J. Nanomat. 2011, 2011, 1-6. [ Links ]
31. Reza Dadfar, A. M., Ramazani A.; Ali Dadafar, S. M. Polymer Composites 2009, 30, 812-819. [ Links ]
32. Hwang, S. J.; Joo Y. L.; Lee, S. J. J. Appl. Polym. Sci. 2008, 110, 141-1450. [ Links ]














