Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.58 no.1 Ciudad de México ene./mar. 2014
Article
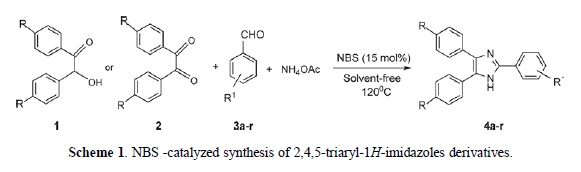
N-Bromosuccinimide Catalyzed Three Component One-Pot Efficient Synthesis of 2,4,5-Triaryl-1H-imidazoles from Aldehyde, Ammonium Acetate, and 1,2-Diketone or α–Hydroxyketone
Behrooz Maleki* and Samaneh Sedigh Ashrafi
Department of Chemistry, Hakim Sabzevari University, Sabzevar 96179-76487, Iran. E-mail: b.maleki@hsu.ac.ir
Received June 10, 2013.
Accepted October 14, 2013.
Abstract
A simple, green, and efficient method for the synthesis of 2,4,5-triaryl-1H-imidazoles using N-bromosuccinimide (NBS) as a catalyst under solvent-free condition is described. The major advantages of the present method are: high yields, less reaction times, solvent-free conditions, easy purification of the products, environmental friendliness, and convenient operation.
Keywords: 2,4,5-Triaryl-1H-imidazoles, aldehydes, ammonium acetate,1,2-diketone, α-hydroxyketone,N-bromosuccinimide.
Resumen
Se describe un método simple, verde y eficiente para la síntesis de 2,4,5-triaril-1H-imidazoles usando N-bromosuccinimida (NBS) como catalizador bajo condiciones libres de disolventes. Las mayores ventajas del método son: altos rendimientos, tiempos de reacción menores, condiciones libres de disolventes, fácilpurificación de productos, condiciones amigables al medio ambiente y operación conveniente.
Palabras clave: 2,4,5-triaril-1H-imidazoles, aldehídos, acetato de amonio, 1,2-dicetona, α-hidroxicetona, N-bromosuccinimida.
Introduction
In 1858 Debus reported the reaction between glyoxal and ammonia, a reaction that pioneered a novel synthetic route to imidazole [1]. Over the century, the importance of imidazoles in biological system has attracted much interest due to their chemical and biochemical properties. Compounds with imid-azole ring system have many pharmacological properties and can play important role in biochemical processes [2-3]. For example, it is reported that substituted imidazoles can act as glucagon receotor anagonists [4], inhibitors of P38 MAP kinase [5], B-Raf kinase [6], plants growth regulators [7], antibacterial [8], antitumour [9], therapeutic agents [10] and also pesticide [11]. In recent years, substituted imidazoles are substantially used in ionic liquids [12-15], that has been given a new approach to "Green Chemistry". The potency and wide applicability of the imidazole phrmacophore can be attributed to its hydrogen bond donor-acceptor capability as week as its high affinity of metals which are present in many protein active sites [16]. Because of their wide rang of pharmacological activity, industrial and synthetic applications, the synthesis of imidaz-oles has received considerable attention, and many articles have appeared. Japp and Radziszewki proposed the first synthesis of the imidazole core in 1822, starting from 1,2-dicarbonyl compounds aldehydes and ammonia, to obtain 2,4,5-triphenyl-1H-imidazole [17-18]. Subsequently, many othesynthesis of this important heterocycle have been published, for example, hetero-Cope rearrangement [19], four-component condensation of arylglyoxals, primary amines, carboxylic acids and isocyanides on wang resin [20], reaction of N-(2-oxo)-amides with ammonium trifluroacetate [21], 1,2-aminalcohols in the presence of PCl5 [22].
In spite of various methods for the synthesis of 2,4,5-triaryl-1H-imidazole generally, these compounds synthesized by three components cyclocondensation of 1,2-diketone or α–Hydroxyketone with an aldehyde and ammonium acetate [23]. Various reagents can catalyze this reactions, such as:H3PO4-12MoO3-24H2O,KH2PO4, [24] catalyst-free under microwave irradiation [25-26], ionic liquid (1-n-butyl and 1,3-di-butyl imidazolium salts) [27], ceric (IV) ammonium nitrate (CAN) [28], Eu(OTf)3 [29], zeolite HY/SiO2 [30], ZrCl4 [31], Yb(OTf)3 [32] NiCl2-6H2O [33], sodium bisulfate [34] iodine [35], sulphanilic acid [36], oxalic acid [37], silica sulfu-ric acid [38], acetic acid [39], L-proline [40], PEG-400 [41], Cu(TFA)2 [42], p-TSA/TBAI [43],(NH4)6Mo7O24-4H2O [44], InCl3-6H2O [45], Zr(acac)4 [46], heteropolyacid [47] and ura-nyl nitrate hexahydrate [UO2(NO3)2-6H2O]supported on acidic alumina [48]. However, many of these methods suffer from longer reaction times, unsatisfactory yields, acidic media, difficult workup, excessive use of reagents and catalyst. It is therefore important to find more convenient methods for the preparation of these compounds.
Results and Discussion
N-bromosuccinimide (NBS) (Fig. 1) has gained interesting attraction in recent years due to economic and environmentally considerations [49-54]. This catalyst is generally inexpensive and easily available, which can conveniently be handled and removed from the reaction mixture. Thus, making a simple and eco-friendly experimental procedure is still strongly desired for the synthesis of these important heterocyclic compounds.

As a part of our program, seeking at development new methodologies for the preparation of heterocyclic compounds containing nitrogen [55-60] herein, we wish to describe a new and convenient protocol for the synthesis of 2,4,5-triaryl-1H-imidazoles via a multicomponent reaction of aldehydes, 1,2-diketone or α–Hydroxyketone, and ammonium acetate in the presence of N-bromosuccinimide under solvent-free conditions (Scheme 1).
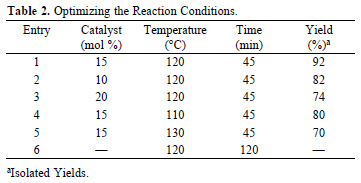
Initially, we investigated the ability of this catalyst for examining the reaction of 4-chlorobenzaldehyde, 1,2-diketone and ammonium acetate. After initial screening of amounts for NBS, solvents and reaction temperature, we obtained that use of 15 mol% NBS at 120 °C under solvent-free conditions produced 2-(4-chlorophenyl)-4,5-diphenyl-1H-imidazole after 45 min, in 92% yield (entry1). Notably, the desired product could not be obtained under similar reaction conditions, even after a long time (120 min) in the absence of the catalyst (entry 6).
Subsequently, to examine the efficiency and applicability of this protocol, the reaction was extended to other substituted benzaldehydes under solvent-free conditions.Importantly for the ultimate goal of applying this reaction in a diversity-generating strategy, this broad generality extends to the l,2-diketone substrate as well (Table l).
A probable mechanism for the synthesis of 2,4,5-triaryl-1H-imidazoles was proposed in Scheme 2. In this procedure, ammonium acetate can be decomposed into ammonia and acetic acid. Ammonia is the nitrogen source. Since NBS contains bromine atom which are attached to nitrogen, it is very probable that this reagent releases Br+ in situ which can conduct as an electrophilic species [48-54]. It can active the carbonyl group (C=O) of aldehyde and decrease the enerdy of transition state. Br+ facilitates the formation of the diimine intermediate [I] that under mild catalysis of NBS (Br+) condenses with the carbonyl carbon of the l,2-diketone followed by dehydration to afford the iso-imidazole which rearranges via a [1,5] sigma-tropic shift to the required 2,4,5-triaryl-1H-imidazoles (4a-1). Using benzoin (1), aromatic aldehydes substrates, and ammonium acetate with NBS as a catalyst, the proposed mechanism includes initial oxidation of Benzoin (2) in the presence of Br+ fllowed by similar mechanism as that for benzil (1) (Scheme 2) [22-47].

Conclusion
In conclusion, the present protocol demonstrates the potential of NBS, as a cheap and readily available reagent, neutral, green and effective catalyst for the synthesis of 2,4,5-triaryl-1H-imidazoles. In this method, complicated operation of pre-separating mixtures is not necessary.
Experimental
Solvents, reagents, and chemical materials were obtained from Aldrich (United States), Merck (Germany) and Fluka (Switzerland) chemical companies and purified prior to use. Melting points were determined in open capillary tubes in a Stuart BI Branstead Electrothermal Cat No:IA9200 apparatus and are uncorrected. Nuclear magnetic resonance spectra were recorded on JEOL FX 90Q using tetramethylsilane (TMS) as an internal standard. IR spectra were recorded on a Shimadzu 435-U-04 spectrophotometer (KBr).
General procedure for the synthesis of 2,4,5-triaryl-1H-imidazoles
To a stirred mixture of the aromatic aldehydes(3a-r) (1 mmol), benzil or benzoin (1 mmol), ammonium acetate (3 mmol), at room temperature was added N-bromosuccinimide (NBS) (15 mol%) and then temperature was raised to 120°C and maintained for the appropriate time (see Table 2). After completion of the reaction (monitored by TLC) the reaction mixture diluted with EtOH (96%, 5 ml) and stirred for 2 min in 120°C. The solvent evaporated, the resulting solid products were collected and washed with water to give the crude products. Then, re-crystallized from EtOH (96%, 5 ml) to afford pure 2,4,5-triaryl-1H-imidazoles (4a-r).
The spectral data for selected compound
2,4,5-Triphenyl 1H-imidazole (4a). Mp 274-275 oC. FTIR (KBr,cm-1): 3451, 2856, 1636, 1490; 1H NMR (400 MHz, DMSO-d6): δ 12.69 (s, 1H), 8.09 (d, 2H), 7.56-7.22 (m, 13H);13C NMR (75 MHz, DMSO-d6): δ 145.6, 137.2, 135.2, 131.2,130.4, 128.7, 128.5, 128.3, 128.2, 127.8, 127.2, 126.6, 125.3.
2-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazole (4b). Mp 264-265 oC. FTIR (KBr,cm-1): 3452, 3065, 1635, 1323; 1H NMR (400 MHz,DMSO-d6): 3 12.78 (s, 1H), 8.11 (d, 2H), 7.56-7.23 (m, 12H); 13C NMR (75 MHz, DMSO-d6): δ 146.3, 130.3, 129.9, 129.2, 128.5, 127.4, 127.0,126.4, 125.5, 125.2, 123.3, 116.3.
2-(3-Nitrophenyl)-4,5-diphenyl-1H-imidazole (4j). Mp>300 oC. FTIR (KBr, cm-1): 3448, 3068, 1526, 1350; 1H NMR (400 MHz, DMSO-d6): δ 13.10 (s, 1H), 8.95 (s, 1H),8.53 (d, 1H), 8.23 (d, 1H), 7.81 (d, 1H), 7.54-7.33 (m, 10H);13C NMR (75 MHz, DMSO-d6): δ 148.4, 143.4, 131.8, 131.2, 130.4, 129.83,128.7, 128.4, 127.1, 122.6, 119.4.
2-(4-Methoxyphenyl)-4,5-diphenyl-1H-imidazole (4k). Mp 227-229 oC. FTIR (KBr,cm-1 ): 3425, 3029, 2956, 1610, 1495, 1249; 1HNMR (400 MHz,DMSO-d6): δ 12.50 (s, 1H), 8.03 (d, 2H),7.50-7.33 (m, 10H), 7.05 (d, 2H), 3.82 (s, 3H); 13CNMR (75MHz, DMSO-d6): δ 159.5, 145.7, 132.24, 131.37, 130.98, 129.34, 128.4, 127.7, 126.8, 123.1, 114.1, 55.2.
Acknowledgments
Authors wish to thank the University of Hakim Sabzevari for financial support to carry out this research. We also thank Mrs. Neda Rahiminezhad for her assistance.
References
1. Debus, H. Liebigs Ann. Chem. 1858, 107, 199. [ Links ]
2. Lambardino, J. G.; Wiseman, E. H. J. Med. Chem. 1974, 17, 1182-1188. [ Links ]
3. Chawla, A.; Sharma, A.; Sharma, A. K. Der Pharm. Chem. 2012, 4, 116. [ Links ]
4. Chang, L.L.; Sidler, K. L.; Cascieri, M. A.; Laszlo, S.; Koch, G.; Li, B.; Maccoss, M.; Mantlo, N.; Okeefe, S.; Pang, M.; Rolando, A.; Hangmann, W. K. Bioorg.Med. Chem. Lett. 2001, 11, 2549. [ Links ]
5. Lee, J. C.; Laydon, J. T.; McDonnell, P. C.; Gallagher, T. F.; Kumar, S.; Green, D.; McNulty, D.; Blumenthal, M. J.; Keys, J. R.; Vatter, S. W. R.; Strickler, J. E.; McLaughlin, M. M.; Siemens, I.R.; Fisher, S. M.; Livi, G. P.; White, J. R.; Adams, J. L.; Young, P. R. Nature 1994, 372, 739. [ Links ]
6. Takle, A. K.; Brown, M. J. B.; Davies, S.; Dean, D. K.; Francis, G. ; Gaiba, A.; Hird, A. W.; King, F. D.; Lovell, P. J.; Naylor, A.; Reith, A. D.; Steadman, J. G.; Wilson, D. M.; Bioorg. Med. Chem. Lett. 2006, 16, 378. [ Links ]
7. Schmierer, R.; Mildenberger, H.; Buerstell, H. German Patent 1987, 361464; Chem. Abstr. 1988, 108, 37838. [ Links ]
8. Antolini, M.; Bozzoli, A.; Ghiron, C.; Kennedy, G.; Rossi, T.; Ursini, A. Bioorg. Med. Chem. Lett. 1999, 9, 1023. [ Links ]
9. Wang, L.; Wooda, K. W.; Li, Q.; Barr, K.J.; Mccroskey, R. W.; Hannick, S. M.; Ghereke, L.; Credo, R.B.; Hui, Y. H.; Marsh, K.; Warner, R.; Lee, J. Y.; Zielinsky-Mozng, N.; Frost, D.; Rosenberg, S. H.; Sham, H. L. J. Med. Chem. 2002, 45, 1697. [ Links ]
10. Heeres, J.; Backx, L. J. J.; Mostmans, J. H.; Van Custem, J. J. Med. Chem. 1979, 22, 1003. [ Links ]
11. Maier, T.; Schmierer, R.; Bauer, K.; Bieringer, H.; Buerstell, H.; Sachse, B. U.S. Patent 1989, 4820335; Chem. Abstr. 1989, 111, 19494w. [ Links ]
12. Hajipour, A. R.; Rafiee, F. J. Iran. Chem. Soc. 2009, 6, 647. [ Links ]
13. Zare, A.; Hasaninejad, A.; Khalafi-Nezhad, A.; Moosavi-Zare, A. R.; Beyzavi, M. H.; Khedri, F.; Asadi, F.; Hayati, N.; Asifi, A. J. Iran. Chem. Soc. 2010, 7, 461. [ Links ]
14. Seo, D. V.; Lim, Y. D.; Lee, S. H.; Ur, S. C.; Kim, V. G. Bull. Korean Chem. Soc. 2011, 32, 2633. [ Links ]
15. Li, H.; Liu, J.; Zhu, J. Wang, H.J. Korean Chem. Soc. 2011, 55, 685. [ Links ]
16. Philips, A.P.; White, H. L.; Rosen, S. Eur. Pat. Appl. EP 1982, 58890. [ Links ]
17. Radziszewski, B. Chem. Ber. 1882, 15, 1493. [ Links ]
18. Japp, F. R.; Robinson, H. H.; Chem. Ber. 1882, 15, 1268. [ Links ]
19. Lantos, I.; Zhang, W. Y.; Shiu, X.; Eggleston, D. S. J. Org. Chem. 1993, 58, 7092. [ Links ]
20. Zhang, C.; Moran, E. J.; Wiowade, T.F.; Short, K. M.; Mjalli, A. M. M. Tetrahedron Lett. 1996, 37, 751. [ Links ]
21. Claiborne, C. F.; Liverton, N. J.;Nguyen, K. T. Tetrahedron Lett. 1998, 39, 8939-8942. [ Links ]
22. Bleicher, K. H.; Gerber, F.; Wuthrich, Y.; Alanine, A.; Capretta, A, Tetrahedron Lett. 2002, 43, 7687-7690. [ Links ]
23. Jadhav, S. D.; Kokare, N. D.; Jadhav,S. D.; J. Het. Chem. 2008, 45, 1461. [ Links ]
24. Joshi, R. S.; Mandhane, P. G.; Shaikh, M. U.; Kale, R. S.; Gill, C. H. ; Chin. Chem. Lett. 2010, 21, 429. [ Links ]
25. Zhou, J. F.; Gong, X. G.; Zhu, H. Q.; Zhu, F. X. Chin. Chem. Lett. 2009, 20, 1198. [ Links ]
26. Zhou, J. F.; Gong, G. X.; Sun, X. J.; Zhu, Y. L. Synth. Commun. 2010, 40, 1134. [ Links ]
27. Siddiqui, S, A.; Narkhede, U. C.; Palimkar, S. S.; Daniel, T.; Lahoti, R. J.; Srinivasan, K. V. Tetrahedron 2005, 61, 3539. [ Links ]
28. Shelke, K. F.; Sapkal, S. B.; Shingare, M. S. Chin. Chem. Lett. 2009, 20, 283. [ Links ]
29. Sangshetti, J.; Kokare, N.; Kotharkara, S.; Shinde, D. J. Chem. Sci. 2008, 120, 463. [ Links ]
30. Yu, C.; Lei, M.; Su, W.; Xie, Y. Synth. Commun. 2007, 37, 3301. [ Links ]
31. Balalaie, S.; Arabanian, A.; Hashtroudi, M. S. Montsh Chem. 2000, 131, 945. [ Links ]
32. Sharma, G. V. M.;, Jyothi, Y.; Lakshmi, P. S. Synth. Commun. 2006, 36, 2991. [ Links ]
33. Wang, L. M.; Wang, Y. H.; Tian, H.; Yao, Y.; Shao, J.; Liu, B. J. Fluorine Chem. 2006, 127, 1570. [ Links ]
34. Heravi, M. M.; Bakhtiari, K.; Oskooie, H. A.; Taheri, S. J. Mol. Catal. A Chem. 2007, 263, 279-281. [ Links ]
35. Sangshetti, J. N.; Shinde, D. B.; Kokare, N. D.; Kotharkar, S. A. Montsh Chem. 2008, 139,125. [ Links ]
36. Kidwai, M.; Mothsra, P.; Bansal, V.; Goyal, R. Montsh Chem. 2006, 137, 1189. [ Links ]
37. Mohammed, A.; Lokare, N.; Sangshetti, J. J. Korean Chem. Soc. 2007, 51, 418. [ Links ]
38. Kokare, N. D.; Sangshetti, J. N.; Shinde, D. B. Synthesis 2007, 18, 2829-2834. [ Links ]
39. Shaabani, A.; Rahmati, A. J. Mol. Catal. A Chem. 2006, 249, 246. [ Links ]
40. Wang, J.; Mason, R.; Derveer, D. V.; Feng, F.; Bu, X. R. J. Org. Chem. 2003, 68, 5415. [ Links ]
41. Samai, S.; Nandi, G. C.; Singh, P.; Singh, M. S. Tetrahedron 2009, 65, 10155-10161. [ Links ]
42. Wang, X. C.; Gong, H. P.; Quan, Z. J.; Li, L.; Ye, H. L. Chin. Chem. Lett. 2009, 20, 44. [ Links ]
43. Song, D.; Liu, C.; Zhang, S.; Luo, D. Synth. React. Inorg. Metal-Org. Nano-Metal Chem.2010, 40, 145. [ Links ]
44. Khodaei, M. M.; Bahrami, K.; Kaviania, I. J. Chin. Chem. Soc. 2007, 54, 829. [ Links ]
45. Safari, J.; Dehgan Khalili, S.; Banitaba, S. H. J. Chem. Sci. 2010, 122, 437. [ Links ]
46. Sharma, S. D.; Hazarika, P.; Konwar, D. Tetrahedron Lett. 2008, 49, 2216-2220. [ Links ]
47. Khosropour, A. R. Ultrason. Sonochem. 2008, 15, 659. [ Links ]
48. Heravi, M. M.; Sadjadi, S.; Oskooie, H. A.; Hekmatshoar, R.; Bamoharram, F. F. J. Chin. Chem. Soc. 2008, 55, 1199. [ Links ]
49. Satyanarayana, V. S. V.; Sivakumar, A. Chem. Papers 2011, 65, 519. [ Links ]
50. Karimi, B.; Zareyee, D. J. Iran. Chem. Soc. 2008, 5, S103. [ Links ]
51. Fujioka, H.; Murai, K.; Ohba, Y.; Hiramatsu, A.; Kita, Y. Tetrahedron Lett. 2005, 46, 2197-2199. [ Links ]
52. Karimi, B.; Seradj, H.; Ebrahimian R. Synlett 2000, 623. [ Links ]
53. Kamal, A.; Chouhan, G. Synlett 2002, 474. [ Links ]
54. Shaterian, H. R.; Yarahmadi, H.; Ghashang, M.; Safari-Mehmandosti, M. Chin. J. Chem. 2008, 26, 2093. [ Links ]
55. Maleki, B.; Azarifar, D.; Ghorbani-Vaghei, R.; Veisi, H.; Hojati, S. F.; Gholizadeh, M.; Salehabadi, H.; Khodaverdian Moghadam, M. Monatsh Chem. 2009, 140, 1485. [ Links ]
56. Ghorbani-Vaghei, R.; Azarifar, D.; Maleki, B. Bull. Korean Chem. Soc. 2004, 25, 953. [ Links ]
57. Zolfigol, M. A.; Azarifar, D.; Mallakpour, S.; Mohammadpoor-Baltork, I.; Forghania, A.; Maleki, B.; Abdollahi-Alibeik, M. Tetrahedron Lett 2006, 47, 833. [ Links ]
58. Azarifar, D.; Zolfigol, M. A.; Maleki, B. Bull. Korean Chem. Soc. 2004, 25, 23. [ Links ]
59. Maleki, B. Coll. Czsch. Chem. Commun. 2011, 76, 27. [ Links ]
60. Maleki, B.; Gholizadeh, M. Sepehr, Z. Bull. Korean Chem. Soc. 2011, 32, 1697. [ Links ]
61. Maleki, B.; Tayebee, R.; Kermanian, M.; Sedigh Ashrafi, S. J. Mex. Chem. Soc. 2013, 57, 298-306. [ Links ]














