Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.57 no.4 Ciudad de México oct./dic. 2013
Article
Zeolite Encapsulated Fe-poprhyrin for Catalytic Oxidation with Iodobenzene Diacetate (PhI(OAc)2)
Gholamreza Karimipour,* Maryam Rezaei, and Davoud Ashouri
Department of Chemistry, Yasouj University, Yasouj. 75918-74831, Iran.
Received February 21, 2013.
Accepted May 5, 2013.
Abstract
Meso-Tetrakis(3-pyridyl)porphyrmatorron(III) chloride encapsulated on NaY Zeolite [Fe(T-3-PyP)@NaY] was synthesized as a heterogeneous "ship-in-a-bottle" type catalyst and characterized by Fourier transform infrared (FT-IR), atomic absorption (AA), diffused reflectance UV-Vis (DR UV-Vis), X-ray diffraction (XRD) and scanning electron microscopy (SEM) analysis. The catalytic activity of Fe(T-3-PyP)@NaY was examined for the epoxidation of cyclohexene by PhI(OAc)2 in CH3CN/H2O (5:1) and compared to that of Fe(T-3-PyP) as a homogenous catalyst. We found that the heterogeneous catalyst Fe(T-3-PyP)@NaY was stable and reusable for several times, and provided a mild condition and exhibited high activity and selectivity in the oxidation of alkenes to epoxides(16-94%). As representative examples for the use of Fe(T-3-PyP)@NaY/ PhI(OAc)2 in organic oxidations, oxidation of 4-nitrobenzylalcohol to 4-nitrobenzaldehyde (97 %), oxidative dehydrogenation of diethyl 4-(2,6-dichlorophenyl)-2,6-dimethyl- 1,4-dihydro-3,5-pyridinedicarboxylate to the corresponding pyridine (100 %), diphenylacetic acid to benzophenone (64 %) was achieved.
Keywords: Metalloporphyrin, Zeolite, Catalyst, Encapsulation, Oxidation.
Resumen
Se sintetizó el catalizador cloruro de raeso-Tetrakis(3-p yridyl)porphyrinatohierro(III) encapsulado en zeolita NaY [Fe(T-3-PyP)@NaY] mediante la técnica "barco en la botella" y se caracterizó por infrarrojo con transformada de Fourier (FT-IR), absorción atómica (AA), UV-vis con reflectancia difusa (DR UV-Vis), difracción de rayos X (XRD) y microscopia electrónica de barrido (SEM). La actividad catalítica de Fe(T-3-PyP)@NaY se examinó mediante la opoxidación de ciclohexeno por PhI(OAc)2 en CH3CN/H2O (5:1) y se comparó con la de Fe(T-3-PyP) como catalizador homogéneo. Encontramos que el catalizador heterogéneo Fe(T-3-PyP)@NaY fue estable y reusable en varias ocasiones, permitiendo condiciones suaves de reacción además de exhibir actividad elevada y buena selectividad en la oxidación de alquenos a epóxidos (16-94%). Citamos como ejemplos representativos del uso de Fe(T-3-PyP)@NaY/ PhI(OAc)2 para las reacciones de oxidación la transformación de 4-nitrobencilalcohol a 4-nitrobenzaldehido (97 %), la deshidrogenación oxidativa de dietil 4-(2,6-diclorofenil)-2,6-dimetil-1,4-dihidro-3,5-piridindicarboxilato a la piridina correspondiente (100 %) y la obtención de benzofenona a partir de ácido difenilacético (64 %).
Palabras clave: Metaloporfirina, zeolita, catalizador, encapsulación, oxidación.
Introduction
Metalloporphyrins are a class of versatile cytochrome P-450 model catalysts which facilitate the oxidation of many organic compounds (C-H bonds activation, epoxidation and hydroxylation) by PhIO, NaIO4, H2O2, NaOCl, PhI(OAc)2, etc. [1]. They are also applied in analytical chemistry, medicine and material science [2]. The first report on the metalloporphyrin-mediated oxidation was given by Groves and co-worker [3] in which PhIO was applied for hydrocarbon oxidation in the presence of iron (III) porphyrin. We have also published some papers in this area which review the nature of the catalysts, oxidants and substrates [1c, 4].
However, a major drawback is the oxidative demolition of porphyrins during their catalytic function, which results in enormously diminishing the oxidation yield. To overcome this imperfection, supporting of metalloporphyrins on an insoluble support has been extensively attempted and many papers have been published on this subject in the last decade [5]. Generally, the immobilization of metalloporphyrins offers several particular advantages over soluble catalysts, such as facilitation of catalyst separation from the reaction mixture, simplification of procedures for catalyst recycling, the possibility of adaption of the immobilized catalyst for a continuous flow process, and obtaining the desired high-yield products.
In the recent years, attention has been focused on zeolites as appropriate supports for encapsulation of metalloporphyrins, due to their molecular-size channels and pores in three-dimensional network of well defined crystalline structures, conferring shape and size selectivity [6]. In general, the aperture size of zeolite pores ranges from 3 to 8 Å, and the inner diameter of interior spaces from 5 to 13 Å [7]. Moreover, zeolites are neutral solids and could be used in organic solvents without any serious implementation problems.
In general, immobilization methods include physical entrapment, covalent binding and surface adsorption [8]. In this work, we prepared a heterogeneous oxidation catalyst through encapsulation of [/weso-tetrakis(3-pyridyl) porphyrinato]iron(III) chloride complex into NaY zeolite (Fig. 1). This complexes (abbreviated as Fe(T-3-PyP)@NaY) is synthesized in the supercage of the zeolite just like building a ship in a bottle; such conformations are often referred to as "ship-in-a-bottle" type catalysts. The heterogeneous catalyst exhibits enhanced stability and a high turnover number, as well as good catalytic activity and selectivity in the epoxidation of alkenes by iodobenzene diacetate (PhI(OAc)2) in organic media.
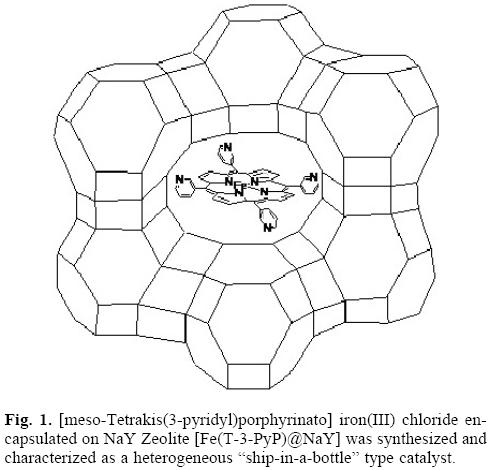
Results and discussions
The iron content of parent NaY zeolite, and Fe(T-3-PyP)@NaY catalyst was measured by dissolving a known amount of the materials in conc. HNO3/HCl (1:4 v/v), from these solutions the iron contents were estimated using atomic absorption spectrometer (AAS). The iron content of NaY zeolite, and Fe(T-3-PyP)@NaY (mmol of iron in gram of zeolite) is equal to ~0.015 mmol/g and ~0.36 mmol/g, respectively. Therefore, the iron content of Fe(T-3-PyP)@NaY was found to be of 24 fold of the neat NaY zeolite, indicating the effective exchange of Na with Fe atoms in NaY zeolite and successful formation of Fe(T-3-PyP) in the zeolite supercages.
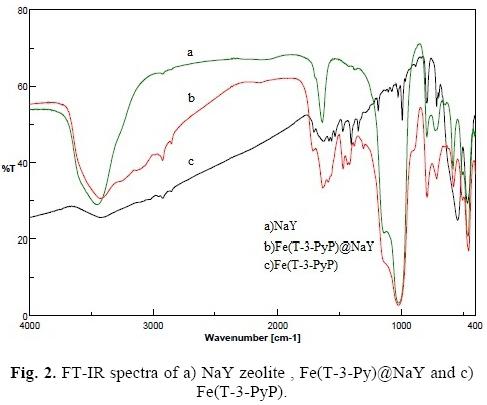
The FT-IR spectra of NaY zeolite, Fe(T-3-PyP) and Fe(T-3-PyP)@NaY are shown in Figure 2. The infrared spectrum of neat NaY (a) shows major bands at 3457, 1639 and 1021 cm-1. The spectrum of Fe(T-3-PyP) (c) is characterized by a moderately broad band at 3417 cm-1 and some weak bands at 1027-1633 cm-1. Upon encapsulation of Fe(T-3-PyP), the bands at 3457 and 1639 cm-1 shifted towards lower frequency at 3430 and 1635 cm-1 correspondingly, which may be due to the interaction of the metal complex with the zeolite matrix (Figure 2, b). Moreover, some weak bands of Fe(T-3-PyP) at 1027-1633 cm-1 (which may be due to the /weso-pyridyl groups) appear again in the Fe(T-3-PyP)@NaY spectrum. While some bands of Fe(T-3-PyP) at 500-1000 cm-1 are detectable in the Fe(T-3-PyP)@NaY spectrum, the infrared spectrum of the encapsulated metalloporphyrin demonstrates no intense bands characteristic of Fe(T-3-PyP). This is the result of the low level of encapsulated Fe-porphyrin load, which guarantees its homogeneous distribution within zeolite cages.
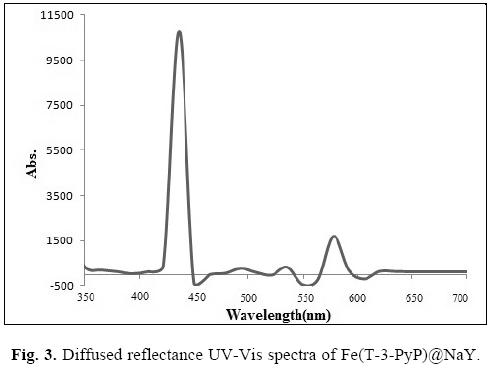
Comparison of UV-Vis spectrum of Fe(T-3-PyP) complex (Fig. 7a, vide infra) and diffused reflectance UV-Vis of Fe(T-3-PyP) encapsulated in zeolite Y (Fig. 3), confirmed the incorporation of metallopophyrin into the supercages of the zeolite. The same band is present in the UV-Vis spectra of Fe(T-3-PyP) entrapped in the NaY Zeolite but the maxima have been shifted to higher wavelength and indicate that the immobilization of the complex modifies the electronic and spectral properties of the encapsulated metalloporphyrin.
The neat NaY zeolite and Fe(T-3-PyP)@NaY were analyzed through powder X-ray diffraction (Fig. 4). The change observed in the relative intensities of the 311 and 220 reflections upon introducing Fe(T-3-PyP) result from the reported case [9] where the exchange of large cations in NaY Zeolite leads to disturbances in the random distribution of small extra framework cations. The change in the location of small cations affects the relative intensities of 311and 220 peaks. Accordingly, the main framework of the zeolite is not damaged and no variation was observed in the zeolite lattice parameters after the exchange and encapsulation procedures.
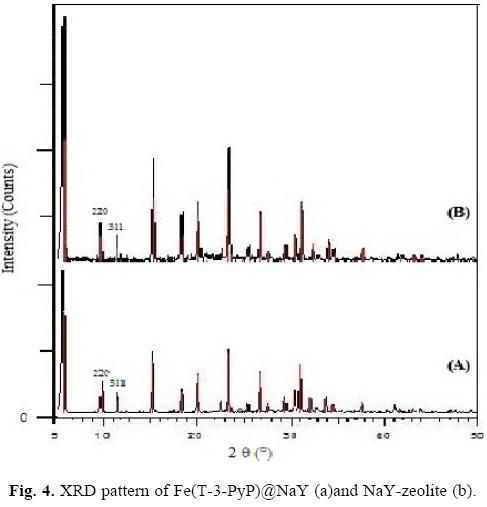
Figure 5, represents the SEM image of NaY-zeolite, Fe@ NaY and Fe(T-3-PyP)@NaY. As it can be seen, these images are similar; indicating that they possess the same morphology, i.e., the framework around the guest molecule Fe(T-3-PyP) was faujasite-Y. These SEM images are very similar to those obtained by other researchers [10]. In general, the SEM image and XRD pattern of Fe(T-3-PyP)@NaY are similar to those observed for NaY zeolite, indicating that they possess the same morphology and crystalline structures, and that the solid supports were structurally unchanged upon encapsulation.
Catalytic oxidation reactions
In general, metalloporphyrins are expensive in view of their synthesis and purification. Moreover, they can be damaged due to degradation of their structure in the reaction mixture. Therefore, encapsulation of metalloporphyrins into natural or industrial supports prevents degradation of the catalysts and enhances their activities and turnover frequencies. In the present study, encapsulation of Fe(T-3-PyP) was achieved by a Na/Fe exchange template procedure followed by template interaction of pyrrole and pyridine-3-carboxaldehyde named as ship-in-a-bottle method. Fe(T-3-PyP) was also prepared as a homogenous catalyst to compare its catalytic activity with Fe(T-3-PyP)@ NaY as a heterogeneous catalyst. The first approach was to attain the optimized condition for epoxidation of alkenes by Fe(T-3-PyP)@NaY (Scheme 1, vide infra). The Fe(T-3-PyP) loading was quantified through measurement of the Fe content in the catalyst by HNO3/HCl digestion of Fe(T-3-PyP)@NaY. The atomic absorption data show that the amount of Fe(T-3-PyP) loaded on NaY zeolite was 2%. Consequently, all the oxidations under study were achieved by 25 mg of Fe(T-3-PyP)@NaY which contains 8.9 x10-3 mmol of Fe(T-3-PyP). To choose the best reaction medium, dichloromethane, ethanol and acetonitrile were applied and sodium meta-periodate (NaIO4), peracetic acid (PAA), m-chloroperoxy benzoic acid (m-CPBA), tetrabutylammonium hydrogen monopersulfate (OXONE®), H2O2, and iodobenzene diacetate (PhI(OAc)2) were employed as oxidants for epoxidation of cyclohexene. We found that the yield was negligible (< 4%) in the absence of the catalyst and no reaction occurred without any oxidant. Moreover, the yield is less than 5% in the presence of Fe(T-3-PyP) as homogenous catalyst and NaIO4, PAA, m-CPBA, OXONE® and H2O2 oxidants. As shown in Figure 6, NaIO4, PAA, m-CPBA, OXONE and H2O2 have very little capability to oxidize cyclohexene into cyclohexene epoxide in CH2Cl2, EtOH and CH3CN in the presence of Fe(T-3-PyP)@NaY. The function of the solvent may depend upon the donor number and dielectric constant which affect the stability of oxo-intermadiate participating in the metalloporphyrin-mediated oxidations [11].
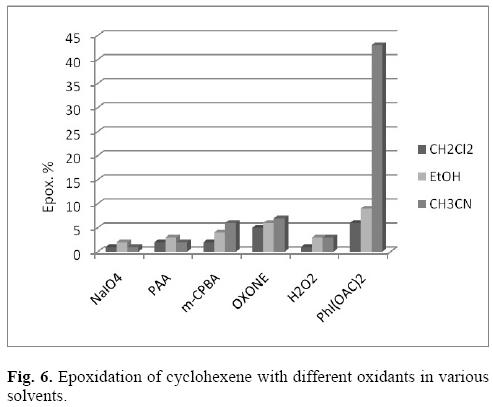
On the other hand, the low yield and low selectivity observed for cyclohexene epoxidation in EtOH may be due to the failure associated with EtOH which can act as a substrate and compete with cyclohexene to make unwanted by-products. However, the catalyst reveals its true role in CH3CN and oxidize cyclohexene with yields as high as 53% by PhI(OAc)2. Therefore, the activity of the catalyst in CH3CN is higher than those in EtOH and CH2Cl2, and it is particularly preferred. It is reasonable that in situ generated PhIO reagent from the reaction of PhI(OAc)2 with water is the efficient oxygen source for the epoxidation (Eq.1) [12].
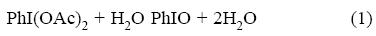
Taking into account the high yield and selectivity, the epoxidation is performed in CH3CN and water using Fe(T-3-PyP)@NaY and PhI(OAc)2 for 2 h at room temperature. As defined above and in the experimental section, 25 mg of Fe(T-3-PyP)@NaY was taken to achieve the oxidation reaction and the optimal molar ratio of Fe(T-3-PyP)@NaY, alkene and oxidant was 1:100:20.
Figure 7 shows the electronic absorption spectra at the end of the reaction (gray line; b) in comparison with Fe(T-3-PyP) itself (black line; a). Absence of the Soret band characteristic of Fe(T-3-PyP) in the mixture at the end of the reaction (spectrum b), indicates that no significant leaching of Fe-porphyrin into the reaction solution occurred during the oxidation.
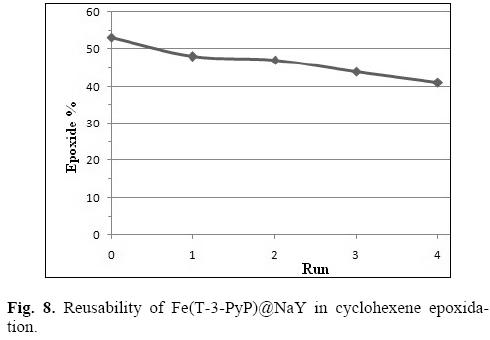
In the second approach, Fe(T-3-PyP)@NaY was easily recovered by phase separation and reused under the same reaction conditions. As shown in Figure 8, the yield of cyclohexene epoxide was still 41% after four reaction cycles, indicating the reusability and validity of Fe(T-3-PyP)@NaY for alkene epoxidation.
The optimal epoxidation conditions employed for cyclohexene were also applied for epoxidation of some other alkenes. It is plausible that the steric and electronic properties of alkene substrates affect the epoxide yields and reaction times.
As shown in Scheme 1, the reactions were found to take place in 16-94 % epoxide yields after stirring at room temperature for 2 h. Exceptionally, styrene (1a) converts into 89 % of styrene epoxide (2a) and 7 % of an unknown byproduct; providing a selectivity of 92%. Surprisingly, indene (1c) and 1-octene (1d) exhibit a lower activity for epoxidation through this catalytic system, affording indene oxide (2c) and 1-octene oxide (2d) in 25% and 16%, respectively. Considering the Fe-porphyrin located in the supercage of the zeolite, explanation of the effect of steric and electronic properties of substrates in the product distribution is not a simple task. A comparison of cyclohexene epoxide obtained by Fe(T-3-PyP) as heterogeneous catalyst with Fe(T-3-PyP)@NaY shows that i) Fe(T-3-PyP) does not show any significant catalytic improvement in total conversion (<5%) and, ii) the turnover number (TON ; the ratio of the number of moles of produced epoxide to the number of moles of catalyst) for the later catalyst was 10.6 times higher than that of the former.
This result confirms that the encapsulation of Fe(T-3-PyP) on NaY-zeolite makes it a more efficient catalyst for oxidation. To investigate the applicability of Fe(T-3-PyP)@ NaY/ PhI(OAc)2, we performed a set of other representative oxidation reactions that are typical of metalloporphyin-mediated reactions. The final products were isolated and their electronic data confirmed their identity with the known compounds. As shown in Scheme 1, oxidation of 4-nitrobenzyl alcohol with Fe(T-3-PyP)@NaY/ PhI(OAc)2 affords 4-nitrobenzaldehyde in 97 % without any over oxidation to 4-nitrobenzoic acid. Accordingly, Fe(T-3-PyP)@NaY/ PhI(OAc)2 provide a mild catalytic oxidation system. Although alcohol oxidation is not generally a difficult job, harsh conditions were necessary for decarboxylation of aryl acetic acids [4a, 13]. However, we can clearly see that this catalytic system promotes adequately decarboxylation of diphenylacetic acid, leading to the formation of benzophenone as a sole product in 64% yield (Scheme 1).
On the other hand, Hantzsch 1,4-dihydropyridines (1,4-DHPs) have received more attention because of their relevant applications in various cardiovascular diseases and hypertension, and their pharmacological activity in antioxidant protective effects [14]. In human body the main metabolic route of dihydropyridine drugs involve their oxidation by cytochrome-450 in the liver [15]. Likewise, oxidation of diethyl 4-(2,6-dichlorophenyl)-2,6-dimethyl- 1,4-dihydro-3,5-pyridinedicarboxylate, as a sample reaction for dehydrogenation of Hantzsch-1,4-DHPs, was achieved in a few minutes by Fe(T-3-PyP)@NaY /PhI(OAc)2 catalytic system with 100% selectivity and yield (Scheme 1). These results point to the catalytic stability, reusability and efficiency of Fe(T-3-PyP)@NaY in combination with PhI(OAc)2, providing a promising catalytic system for oxidation of a wide range of materials .
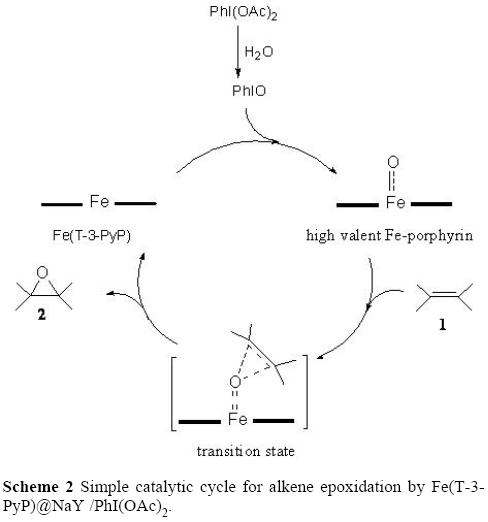
Cytochrome P450 is a heme-containing enzyme that uses dioxygen to incorporate one oxygen atom into organic substrates. Clearly, the characterization of the mechanism that ensures the oxygen transfer into substrates constituents a major task for present research in the field of biomimetic chemistry. Today, it is accepted that a high-valent iron(IV)-oxo intermediate is responsible for the in-vivo oxidation of drugs and xenobiotics. This high valent iron(IV)-oxo intermediate and probably other intermediates of the P-450 catalytic cycle can be formed by the reaction of iron(III) porphyrins with different monooxygen donors [1a, 16]. Therefore, cytochrome P-450 enzymes and their synthetic mimics (i.e. metalloporphyrins) are potent catalysts that are able to catalyze the hydroxylation of saturated carbon hydrogen bonds, the epoxidation of double bonds, the oxidative dealkylation reactions of amines, oxidations of aromatics, and the oxidation of heteroatoms [1b, 17]. From the mechanistic point of view, PhIO was generated in situ by the reaction of PhI(OAc)2 with H2O (Eq.1; vide supra). It is thus inevitable that the high valent Fe-oxo porphyrin will be produced in the second stage by the action of PhIO and the immobilized Fe(T-3-PyP), as described by Groves and others [3, 18]. In the reaction mixture interaction of the high valent Fe-oxo porhyrin with substrates (i.e alkene 1) passes through a transition state and results in oxidation of the substrates to give the corresponding oxidation products (i. e. epoxides 2) (Scheme 2).
In the present study, meso-tetrakis(3-pyridyl)porphyrina toiron(III) chloride complex; Fe(T-3-PyP), was immobilized in NaY-zeolite using template ship-in-a-bottle method and the synthesized sample (Fe(T-3-PyP)@NaY) was characterized by various spectroscopic techniques. Both the Fe(T-3-PyP) and Fe(T-3-PyP)@NaY) as homogenous and heterogeneous catalysts respectively, were used for alkene epoxidation with PhI(OAc)2 in the presence of water. The results show that Fe(T-3-PyP)@NaY catalyst is more efficient than Fe(T-3-PyP), because of (i) catalyst recovery and reusability, (ii) lower level of leaching from the support (iii) mild conditions with easy workup of the products and (iv) high turnover number. The present catalytic system is efficient enough to oxidize various alkenes, alcohols, arylacetic acids and Hantzsch 1,4-dihydropyridines with high to excellent yield and selectivity.
Experimental
Materials
All reagents were of commercial reagent grade and used without further purification. FeCl2.4H2O (Sigma) was employed for zeolite ion exchange. Methanol, ethyl acetate, n-hexane, dichloromethane and acetonitrile were treated and distilled with specific reference to the procedures described in the relevant literature [19]. Propionic acid and pyrrole (Aldrich) were used freshly distilled. All solvents were stored over activated 3 or 4 Å molecular sieves (Aldrich). The NaY zeolite was purchased from Sigma Aldrich. Powder X-ray diffraction (XRD) measurements were performed using Bruker D8 Advance diffractometer with a scanning range of 2θ scale of 5-80° using Cu/K-radiation (X = 1.5406 A° , kV = 40, mA = 40). Atomic absorption spectra (AAS) were recorded on a Varian-240 spectrophotometer using a flame approach, after acid (HNO3/HCl) dissolution of known amounts of the zeolitic materials. Diffuse Reflectance UV-Visible spectra (DR UV-Vis) were recorded by an Ava Spec-2048TEC spectrometer (Light Source: AvaLight DH-S (Just for DRS spectrum). UV-vis spectra were recorded on a Jasco V-530 spectrophotometer and the FT-IR spectra were evidenced in the range of 400-4000 cm-1 using a Jasco FT-IR-460 plus spectrophotometer, in KBr pellets containing zeolitic compounds or free metalloporphyrins. The micrographs from scanning electron microscopy (SEM) were obtained using a Philips XL-30 microscope equipped with an EDS system. The products were initially identified by a comparison with authentic specimens and analyzed by GLC(6890N Agilent; 1.5 m of 10% SE-on packed column, FTIR and 1H NMR (250 MHz, Bruker, CDCl3, TMS) spectroscopy.
Preparation of 5,10,15,20-tetra (3-pyridyl) porphyrin (T-3-PyP) and iron 5,10,15,20-tetra (3-pyridyl) porphyrin [Fe(T-3-PyP)].
T-3-PyP was prepared according to the procedure described by Zilio via condensation of freshly distilled pyrrole with pyridine-3-carboxaldehyde [20]. The iron porphyrin Fe(T-3-PyP) was prepared in refluxing dimethylformamide in the presence of ferrous chloride according to the procedure of Adler [21]. The porphyrin and its iron complex were purified by column chromatography on alumina or silica gel using chloroform/ ethanol (1:3 v/v) as eluent.
Preparation of Fe@NaY
The Fe-exchanged NaY zeolite (Fe@NaY) is air sensitive and its synthesis requires air-free techniques. So, the exchange procedure was carried out under nitrogen atmosphere using deionized, boiled and degassed distilled water. In a three neck vessel installed inside a glove bag, 8.0 g of NaY zeolite was left under nitrogen stream, stirred with 300 mL of H2O and 0.15 ml drops of concentrated HCl for 2 h [22]. The Fe(II) solution was prepared using 1.6 g of FeCl2.4H2O with 160 mL of degassed H2O and treated with 0.1 ml of concentrated HCl. This solution was then added to the NaY system and the mixture was stirred for 12 h always under dynamic nitrogen atmosphere. The mixture was filtered, washed and the solid was transferred to a degassed tube and dried in an Abderhalden apparatus. The color of the solid changed from white-green to light yellow in the regular atmosphere during 5-10 h. So, it should be kept under nitrogen or argon in tightly closed vessels to prevent oxidation of the absorbed Fe(II) ions.
Preparation of Fe(T-3-PyP)@NaY
A suspension of 2.0 g of fresh Fe@NaY, 60 mL of propionic acid, 1.0 mL of freshly distilled pyrrole and 1.50 mL of pyridine-3-carboxaldehyde were placed in round-bottomed three neck flask fitted with septum, reflux condenser and nitrogen inlet port. The mixture was stirred under a nitrogen atmosphere, in a glycerin bath at 135-140 °C, for 24 h. The suspension was centrifuged and the solid obtained was washed by a Soxhlet extraction in dichloromethane and methanol (duration of 100 h or more) until the eluent was colorless.
Catalytic oxidation reaction
Catalytic oxidation reactions were carried out in a 2 ml round bottom flask equipped with a magnetic stirrer at room temperature. In a standard experiment within the reactor, alkene (0.89 mmol) and Fe(T-3-PyP)@NaY (25 mg, containing 8.9 × 10-3 mmol of Fe(T-3-PyP) were suspended in 1.25 ml of acetonitrile containing 0.25 ml of water. The oxidant (PhI(OAc)2; 0.057 g; 0.178 mmol) was then added and the oxidation reaction was carried out under magnetic stirring for the required time. Small aliquots of the reaction mixture were then separated from the zeolitic solid and the resulting solution was directly analyzed by GLC (Agilent 6890N, with SE-30 capillary column). No products were detected when the catalyst used was NaYzeolite or Fe@NaY. Similar reaction conditions were also applied for the alcohol, arylacetic acid and 1,4-dihydropyridin (1,4-DHP) oxidations to evaluate the oxidation capability of the catalyst.
Acknowledgements
The financial support of this study by Yasouj University Research Council is acknowledged.
References
1. a) Meunier, B.; de Visser, S. P.; Shaik, S. Chem. Rev. 2004, 104, 3947-3980; [ Links ] b) Meunier. B. Chem. Rev. 1992, 92, 1411-1456; [ Links ] (c) Mohajer, D.; Karimipour, G.; Bagherzadeh, M. New. J. Chem. 2004, 28, 740-747. [ Links ]
2. a) Feese, E.; Sadeghifar, H.; Graze, H. S.; Argyropoulos, D. S.; Ghiladi. R. A. Biomolecules, 2011, 12, 3528-3539; [ Links ] b) Biesaga, M.; Pyrzynska, K.; Trojanowicz, M. Talanta. 2000, 51, 209-224. [ Links ]; c) Suslick, K. S.; Rakow, N. A.; Kosal, M. E.; Chou, J-H. J. Porphyrins Phthalocyanines 2000, 4, 407-413. [ Links ]
3. Groves, J. T.; Nemo, T. E.; Myers, R. S. J. Am. Chem. Soc. 1979, 101, 1032-1033. [ Links ]
4. a) Karimipour, G.; Karami, B.; Montazerozohori, M.; Zakavi, S. Chin. J. Catal. 2007, 28, 940-946. [ Links ]; b) Karimipour, G.; Montazerozohori, M.; Karami. B. J. Chem. Res. 2006, 605-608. [ Links ]
5. a) Viana, I. L.; Manso, C. M. C. P.; Serra, O. A.; Iamamoto, Y. J. Mol. Catal. A. 2000, 160, 199-208. [ Links ]; b) Benedito, F. L.; Nakagaki, S.; Saczk, A. A.; Peralta-Zamora, P. G.; Costa, M. C. M. Appl. Catal. A. 2003, 250, 1-11; [ Links ] c) Hibino, T.; Jones, W. J.Mater. Chem. 2001, 11, 1321-1323. [ Links ]; (d) Madadi, M.; Rahimi, R. Reac. Kinet. Mech. Cat. 2012, 107, 215-229. [ Links ]
6. a) Poltowicz, J.; Pamin, K.; Tabor, E.; Haber, J.; Adamski, A.; Sojka, Z. Appl. Catal. A. 2006, 299, 235-242. [ Links ]; b) Hutchings, G. J. Chem. Commun. 1999, 4, 301-306. [ Links ]; c) Nakagaki, S.; Xavier, C. R.; Wosniak, A. J.; Mangrich, A. S.; Wypych, F.; Cantao, M. P.; Denicolo, I.; Kubota, L. T. J. Colloid Surf. A. 2000, 168, 261-276. [ Links ]; d) Suib S. L. Chem Rev. 1993, 93, 803-826. [ Links ]
7. Yoon, K. B. Chem. Rev. 1993, 93, 321-339. [ Links ]
8. a) Biazzotto, J. C.; Sacco, H. C.; Ciuffi, K. J.; Neri, C. R.; Ferreira, A. G.; Iamamoto, Y.; Serra, O. A. J. Non-Cryst.Solids. 1999, 247, 134-140. [ Links ]; b) Iamamoto, Y.; Sacco, H. C; Biazzotto, J. C; Ciuffi, K. J. An. Acad. Bras. Ci. 2000, 72, 59-66. [ Links ]; c) Sacco, H. C.; Biazzotto, J. C.; Ciuffi, K. J.; Serra, O. A.; Nascimento, O. R.; Zuchi, M. R.; Leite, C. A. P.; Iamamoto, Y. J. Non-Cryst. Solids. 2000, 273, 150-158. [ Links ]
9. Ahmed, A. H.; Mostafa, A. G. Material Science and Engineering C. 2009, 29, 877-883. [ Links ]
10. Nakagaki, S.; Xavier, C. R.; Wosniak, A. J.; Mangrich, A. S.; Wypych, F.; Cantao, M. P.; Denicolo, I.; Kubota, L. T. Colloids and surfaces A: Physicochemical and Engineering 2000, 168, 261-276. [ Links ]
11. a) Takahashi, A.; Kurahashi, T.; Fujii, H. Inorg. Chem. 2011, 50, 6922-6928. [ Links ]; b) Drazen O., Bruice T. C. Acc. Chem. Res. 1992, 25, 314-320. [ Links ]
12. In. J. H.; Park, S. E.; Song, R.; Nam, W. Inorg. Chim. Acta 2003, 343, 373-376. [ Links ]
13. Tanner, D. D.; Osman, S. A. A. J. Org. Chem. 1987, 52, 46894693. [ Links ]
14. a) Oike, M.; Inoue, Y.; Kitamura, K.; Kuriyama, H. Circ. Res. 1990, 67, 993-1006. [ Links ]; b) Yamamoto, T.; Niwa, S.; Ohno, S.; Oni-shi, T.; Matsueda, H.; Koganei, H.; Uneyama, H.; Fujita, S.-i.; Takeda, T.; Kito, M.; Ono, Y.; Saitou, Y.; Takahara, A.; Iwata, S.; Shoji, M. Bioorg. Med. Chem. Lett. 2006, 16, 798-802. [ Links ]
15. Guengerich, F. P.; Martin, M. V.; Beaune, P. H.; Kremers, P.; Wolff, T.; Waxman, D. J. J. Biol. Chem. 1986, 261, 5051-5060. [ Links ]
16. (a) Tshuva, E. Y.; Lippard, S. J. Chem. Rev. 2004, 104, 987-2011. [ Links ]; (b) Costas, M.; Mehn, M. P.; Jensen, M. P.; Que, L., Jr. Chem. Rev. 2004, 104, 939-986. [ Links ]
17. (a) Meunier, B. Biomimetic Oxidations Mediated by Metal Complexes; Imperial College Press:London, 2000; [ Links ] b) Meunier, B. Me-talloporphyrin Catalyzed Oxidation; Kluwer Academic Publisher, 1994. [ Links ]
18. Kang, M. J.; Song, W. J.; Han, A. R.; Chi, Y. S.; Jang. H. G.; Nam, W. J. Org. Chem. 2007, 72, 6301-6304. [ Links ]
19. Perrin, D. D.; Armarego, W. L. F.; Perrin, D. R. Purification of Laboratory Chemicals, Pergamum Press, Elmsford, NY, 2nd ed, 1980. [ Links ]
20. Neto, N. M. B.; Boni, L. De.; Rodrigues, J. J.; Misoguti, L.; Mendonça, C. R.; Dinelli, L. R.; Batista, A. A.; Zilio, S. C. J. Porphyrins Phthalocyanines 2003, 7, 452-456. [ Links ]
21. Adler, A. D.; Longo, F. R.; Finarelli, J. D.; Goldmacher, J.; Assour. J.; Korsakoff, L. J. Org. Chem. 1967, 32, 476. [ Links ]
22. Pearce, J. R.; Mortire, W. J.; Uytterhoeven, J. B.; Lunsford, J. H. Faraday Trans. 1981, 177, 937. [ Links ]














