Services on Demand
Journal
Article
Indicators
Related links
Share
Journal of the Mexican Chemical Society
Print version ISSN 1870-249X
J. Mex. Chem. Soc vol.57 n.3 Ciudad de México Jul./Sep. 2013
Article
Electrochemical Study of the Complex [Cu(pdto)(H2O)]2+ (pdto =1,8-bis(2-pyridyl)-3,6-dithiaoctane) in the Presence of the Superoxide. Toward an Electrochemical Method to Measure SOD Activity
Mayra Manzanera-Estrada,1 Luis Felipe Hernández Ayala,2 Guadalupe Osorio-Monreal,2 Juan Carlos García-Ramos,2 and Luis Ortiz-Frade*1
1 Electrochemistry Department. Centro de Investigación y Desarrollo Tecnológico en Electroquímica S.C. Parque Tecnológico Querétaro, Sanfandila, Pedro de Escobedo, C.P. 76703. Querétaro, México. lortiz@cideteq.mx
2 Departamento de Química Inorgánica y Nuclear, Facultad de Química, Universidad Nacional Autónoma de México, Av. Universidad 3000, Ciudad Universitaria, México, D.F., 04510, México.
Received February 27, 2013
Accepted June 5, 2013
Abstract
This work presents the electrochemical characterization of Cu(NO3)2•2.5H2O, [Cu(pdto)H2O](PF6)2 and superoxide ion in DMSO. The electrochemical processes Cu(II) + 2e → Cu(0), [Cu(pdto)(H2O)]2+ + 1e → [Cu(pdto)]+, [Cu(pdto)]+ + 1e → Cu(0), O2 ↔ O2 + 1e-, and O2•- + 1e + H+ → OOH-were identified. The electrochemical response of a mixture of the complex [Cu(pdto)(H2O)]2+ with superoxide was studied and, according to this result, it was possible to establish a simple methodology that indicates if a compound presents a superoxide dismutase (SOD)-like mechanism.
Keywords: Copper (II) complex, pdto=1,8-bis-(2-pyridyl)-3,6-dithiaoctane, SOD mechanism, electrochemistry.
Resumen
En este trabajo se presenta la caracterización electroquímica de Cu(NO3)2•2.5H2O, [Cu(pdto)H2O](PF6)2 y del ion superóxido en DMSO. Se identificaron los procesos electroquímicos Cu(II) + 2e → Cu(0), [Cu(pdto)(H2O)]2+ + 1e → [Cu(pdto)]+, [Cu(pdto)]+ + 1e → Cu(0), O2•-↔ O2 + 1e-y O2•-+ 1e + H+ →OOH-. Posteriormente, se estudió la respuesta electroquímica de una mezcla del complejo [Cu(pdto)(H2O)]2+ con el ion superóxido y de acuerdo con este resultado, fue posible establecer una metodología sencilla que indica si un compuesto presenta un mecanismo tipo superoxido dismutasa, SOD.
Palabras clave: Complejos de Cu(II), pdto=1,8-bis-(2-piridil)-3,6-ditioctano, mecanismo SOD, electroquímica.
Introduction
Intracellular enzyme superoxide dismutase (SOD) in normal conditions, has the role of protecting the cell against oxygen radicals generated in the processes of cellular respiration. SOD enzyme transforms two superoxide radicals to hydrogen peroxide and molecular oxygen. Its active site is a coordination compound with transition metals, such as Cu, Zn, Fe, Mn or Ni, depending on the place where the biosynthesis takes place, with homologies in their sequences and three-dimensional structure [1, 2]. Several studies have proposed that a decrease in Mn-SOD and Cu-Zn SOD activities is one of the most important differences between normal cells and tumor cells [3-5]. The role of ZnCu-SOD is manifested in a significant survival of mice with solid tumors, after a single administration dose (IV/IM) of this enzyme [6]. The above-mentioned have attracted attention to design new SOD biomimetic compounds [7-13]. The catalytic reaction mechanism that presents the ZnCu-SOD, and therefore a biomimetic compound, consists in a disproportion of superoxide radical in two steps, first to generate molecular oxygen, O2, and subsequently the formation of hydrogen peroxide, H2O2, see equations 1 and 2. It has been proposed that for a SOD biomimetic complex, its formal electrode potential (Cu2+L/Cu+L) should be ranging from -330 to 890 mV/NHE at pH = 7.0, [14, 15].
Cu2+L + O2- → Cu+L + O2 (1)
Cu+L + O2- + 2H+ → Cu2+L + H2O2 (2)
The aqueous formal electrode potential value for the complex [Cu(pdto)(H2O)]2+ (pdto = 1,8-bis-(2-pyridyl)-3,6-dithiaoctane) see figure 1, and its interaction with DNA, led to our group to explore the biological activity of this complex with several tumor lines, finding a similar activity than those obtained for the compound cis-platin in the tumor line Hela [16-20]. However, in this study the reaction between the copper complex and the superoxide ion was not studied.
A widely used test for determining if a compound presents a SOD-like mechanism is the Xanthine oxidase bioassay. In the standard procedure the Xanthine/Xanthine oxidase system is used as a source of superoxide radicals and the native cytochrome c is used as a radical trapping. When the SOD enzyme is added in the assay, the reduction of cytochrome c presents a decrease, due to the disproportion of superoxide by the enzyme [21]. The reaction between superoxide and cytochrome c is followed spectrophotometrically at 550 nm. To evaluate the SOD activity, the enzyme is replaced by a compound of interest. The change in absorbance represents the percentage transformed, with the use of a plot concentration vs. volume, a value of SOD activity is obtained and referenced to the activity of the SOD enzyme [9, 22]. However, this method has several disadvantages which include the use of expensive reagents, highly controlled conditions, and interferences associated to interactions between the compound of interest with the radical generator (xanthine/xanthine oxidase) and the radical trapping (cytochrome c) [21]. These interferences can be avoided by using dimethylsulfoxide in alkaline conditions, which promote the generation of superoxide radicals [23]. Furthermore, in this bioassay the short half-life of the superoxide in aqueous solution is not considered, thus the values of SOD activity are under estimated. Several electrochemical methods have been proposed for SOD activity measurements, based in the generation of superoxide ion by autoxidation of pyrogallol, 6-hydroxy-dopamine or O2 [24, 25]. These methods present disadvantages, such as: being an indirect way to detect and to generate the superoxide ion, the presence of parallel reactions, the use of surfactants and the use of hanging drop mercury electrode (HDME). Therefore, it is necessary to find alternative methods that show evidence of a SOD-like mechanism, considering the stability of the superoxide and with easy implementation in laboratory. According to the above-mentioned, this work presents the electrochemical response of Cu(NO3)2•2.5H2O and Cu(II)-(pdto) in a solvent that stabilizes the superoxide ion, such as DMSO, in order to study the reaction between the complex and superoxide using simple electrochemical methods. The results obtained in this work allowed us to establish the feasibility of using simple electrochemical techniques to measure SOD activity.
Results and Discussion
Spectroscopic and electrochemical characterization in DMSO of Cu(NO3)2•2.5H2O
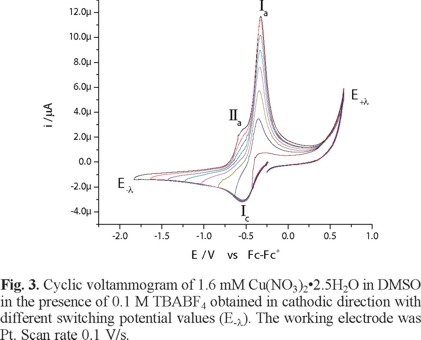
The electronic spectrum of Cu(NO3)2•2.5H2O in DMSO shows a broad signal with maximum wavelength at 840 nm, attributed to an electronic transition d-d, with a molar extinction coefficient value of 27 Lmol-1cm -1 [26]. Figure 2 shows the voltammetric response, recorded in cathodic direction, of 1.6 mM Cu(NO3)2•2.5H2O in DMSO, in the presence of 0.1 M TBABF4 as supporting electrolyte. In this experiment, one reduction process Ιc, with a peak potential value Epc(Ι) = -0.538 V/Fc-Fc+, was observed. When the potential scan was inverted to anodic direction, two oxidation signals, ΙΙa and Ιa, are detected with peak potential values Epa(ΙΙ) = -0.566 V/Fc-Fc+ and Epa(Ι) = -0.323 V/Fc-Fc+. The low oxidation barrier near 0.6 V V/Fc-Fc+ is indicative of the presence of water from the copper salt. When the experiments were performed with a dehydrated copper salt, the oxidation barrier was observed near 1 V/Fc-Fc+, experiments not shown. The immediate increase in cathodic current when the experiment was initiated could be related to the presence of two chemical species from different redox couples, being one of these the chemical species of Cu(II) that generate a mixed potential. Figure 3 presents a series of cyclic voltammograms with different switching potential values (E-λ) in cathodic direction. As can be seen from this figure, the current associated to the processes Ιa and ΙΙa present an increase at switching potential values (E-λ) near to potentials where the reduction process Ιc is recorded; therefore, dependence between reduction and oxidation processes can be established.
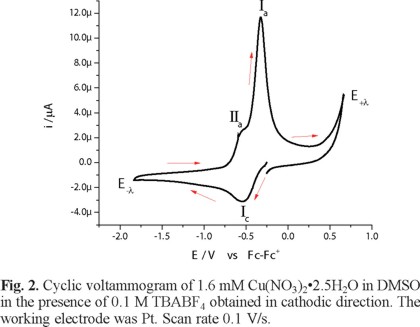
The process Ιc corresponds to the electrochemical reaction Cu(II) + 2e → Cu(0), while processes Ιa and ΙΙa can be associated with two copper stripping reactions, each one for a different chemical species of Cu(II) [27].
Study of the metal complex formation Cu(II)-pdto in DMSO
In order to study the formation of coordination compound Cu(II)-pdto in DMSO, spectroscopic and electrochemical experiments were carried out. Figure 4 shows typical UV-Vis spectra of 2.5 mM Cu(NO3)2•2.5H2O in DMSO in the presence of different amounts of pdto. This figure shows two electronic transitions at 840 nm and 584 nm, related to a Cu(II) complex with a Jahn Teller distortion [26].
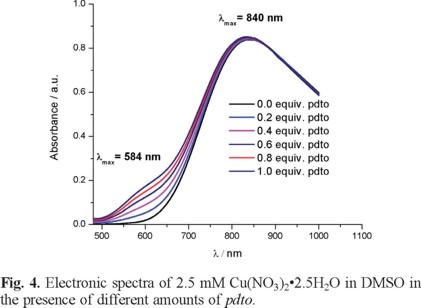
The spectroscopic experiments allowed us to establish the formation of the metal complex. However, this response did not indicate the stabilization of Cu(I) and Cu(II) species, which is the first step to propose a SOD like mechanism. Therefore, electrochemical experiments were carried out. Figure 5 presents typical cyclic voltammograms of 2.5 mM Cu(NO3)2•2.5H2O in DMSO + 0.1 M TBABF4 in the presence of different equivalents of pdto. It is observed that the addition of pdto decreases the current associated with the processes Ιc, Ιa and ΙΙa. Simultaneously, three new signals Ι'c, ΙΙ'c and ΙΙ'a are observed, with peak potential values at -0.250, -0.970, and -0.626 V/Fc + Fc, respectively.

These results indicate that the chemical species [Cu(pdto)]+ is stabilized at the electrode interface as a consequence of the flexibility of the pdto ligand towards the preferential geometry of the central atom, such as in their corresponding Cu (II) and Cu(I) complexes reported in literature [17]. Hence, it is possible to propose the next reduction processes:
[Cu(II)-(pdto)L]2+ + 1e → [Cu(I)-(pdto)]+ +L L=DMSO or H2O I'c
[Cu(I)-(pdto)]++ 1e → Cu(0) II'c
The anodic processes Ιa, ΙΙa and ΙΙ'a correspond to the stripping of Cu(0). The shift to more negative peak potential value for signal ΙΙ'a compared with ΙΙa is caused by the formation and stabilization of [Cu(pdto)]+ in the redissolution process. A detailed study of these signals is beyond the scope of this paper.
In situ reaction between Cu(NO3)2•2.5H2O and pdto in DMSO release water from the copper salt to the medium, making the superoxide ion unstable and causing a low oxidation barrier. Therefore, we decided to obtain the corresponding complex [Cu(pdto)(H2O)](PF6)2, which contains the minimum amount of water, to study its reaction with the superoxide ion.
Electrochemical behavior of the compound [Cu(pdto)H2O](PF6)2
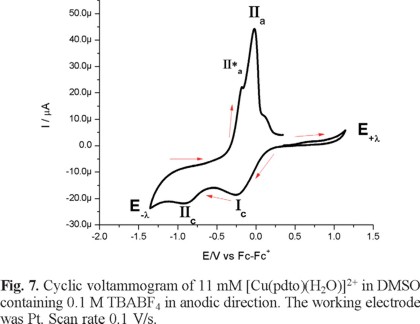
Figure 6 presents a cyclic voltammogram, obtained in cathodic direction, for a 1mM solution of [Cu(pdto)(H2O)](PF6)2 in DMSO containing 0.1 M TBABF4 as supporting electrolyte. In the whole potential scan two reduction processes, Ιc and ΙΙc, and three main oxidation processes, ΙΙ*a, ΙΙa and Ιa, were observed; with their corresponding values Epc(Ι) = -0.249 V/Fc-Fc+, Epc(ΙΙ) = -0.846 V/Fc-Fc+, Epa(ΙΙ*a) = -0.111 V/Fc-Fc+, Epa(ΙΙa) = -0.015 V/Fc-Fc+ and Epa(Ιa) = 0.649 V/Fc-Fc+. When the experiment was performed in anodic direction, see figure 7, the same signals were observed, with the exception of signal Ιa. This last signal indicates the stabilization of a copper (I) species generated after the copper (0) stripping, due to the low content of water in the solution.
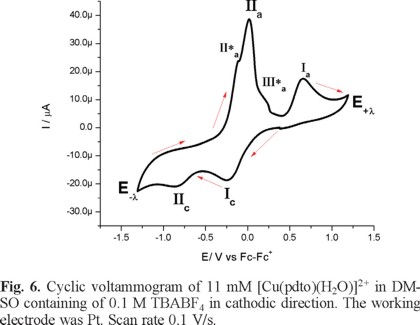
Considering the facultative nature of pdto, already mentioned, it is possible to propose for the principal reduction processes the following electrochemical reactions with their corresponding values of half wave potential E1/2:
[Cu(pdto)(H2O)]2+ + 1e → [Cu(pdto)]+ E1/2 = -0.047V vs Fc-Fc+ Ic'
[Cu(pdto)]+ + 1e →Cu(0) E1/2 = -0.735 V vs Fc-Fc+ IIc'
The anodic processes, ΙΙ*a, ΙΙΙ*a, and ΙΙa can be also related with the formation of different chemical species from the copper stripping processes. Electrochemical Quartz Crystal Microbalance (EQCM) should be performed in order to confirm this idea, but this is beyond the scope of this paper.
Electrochemical characterization of superoxide in DMSO
Figure 8 shows the voltammetric response of superoxide O2 •-(KO2 + 2 equiv. of 18-crown-6 ether) in DMSO containing supporting electrolyte, recorded in anodic direction. It can be observed one oxidation process, Ιa, and two reduction processes, Ιc and ΙΙc, with peak potential values Epa(Ιa) = -0.856, Epc(Ιc) = -1.073 and Epc(ΙIc) = -1.596 V/Fc-Fc+. The absence of the corresponding oxidation signal for ΙΙc suggests a EC mechanism O2 •-+ 1e + H+ →OOH-[27,28]. Water traces in the experimental conditions could be the source of proton donor in the coupled reaction.

Additionally, the voltammetric response of superoxide O2•- (KO2 + of 2 equiv. of 18-crown-6 ether) in cathodic direction was recorded, see figure 9. In this case one reduction process, ΙΙc (Epc = -1.561 V/Fc-Fc+), and one oxidation process, Ιa (Epa = -0.818 V/Fc-Fc+) are observed. This fact confirms the chemical reaction coupled in process ΙΙc and indicates that their products affect the process Ι.

Based on the literature, process Ι is attributed to the electrochemical reaction O2 ↔ O2 + 1e-(E° = -0.965 V/Fc-Fc+), while ΙΙ corresponds to the irreversible process O2 + 1e + H+ →OOH-[28]. In order to establish the stability of the superoxide solutions, voltammetric experiments were recorded as a function of time. The results shown that there are not significant changes in peak potential values and current peak values for processes Ι and ΙΙ (experiment not shown for simplicity) during three hours, indicating that the ion O2 is stable in this time window.
Electrochemical response of the reaction of complex [Cu(pdto)(H2O)]2+ with superoxide
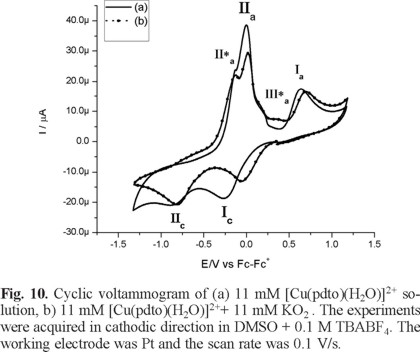
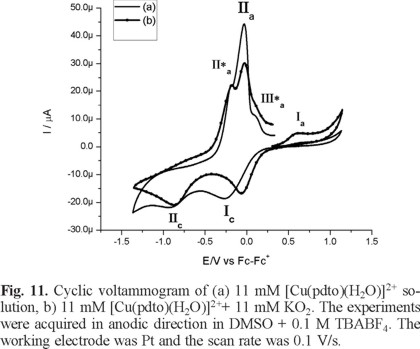
Figure 10 and Figure 11 show typical cyclic voltammograms in cathodic and anodic directions of [Cu(pdto)(H2O)]2+ and its mixture with the superoxide ion (O2 •-). An inspection of the reaction mixture voltammogram allow us to see a displacement in the peak potential value for process Ιc, and a decrease in the current for processes ΙΙa and ΙΙa*. Additionally, the signals related to superoxide after the reaction mixtures are not observed. On the other hand, when the experiments were performed in anodic direction, the current Ιa associated with the presence of [Cu(pdto)]+ in solution was detected, figure 11. This species was generated from the homogeneous electron transfer between the complex [Cu(pdto)(H2O)]2+ and the superoxide ion, according to their respective values E1/2 = -0.047 V/Fc-Fc+ for process [Cu(pdto)(H2O)]2+ + 1e → [Cu(pdto)]+ and E° = -0.965 V/Fc-Fc+ for the process O2 ↔ O2 + 1e. These values also suggest that the coordination compound did not present a catalytic SOD mechanism. The presence of the species [Cu(pdto)]+ and the shift in the peak potential value for signal Ιc, attributed to chemical species with different coordination sphere, probably [Cu(pdto)(OH)]+ or [Cu(pdto)O2 •-]+ confirm this idea. The results obtained in this work encourage us to study a system with a well-known SOD-like activity in a future paper.
Conclusions
By using cyclic voltammetry it was possible to study the electrochemical response of Cu(NO3)2•2.5H2O and [Cu(pdto)(H2O)]2+ in a solvent that stabilizes the superoxide ion as DMSO. The facultative nature of the ligand pdto, allowed us to observe the processes [Cu(pdto)(H2O)]2+ + 1e → [Cu(pdto)]+ and [Cu(pdto)]+ + 1e → Cu(0) in DMSO. It was established that the metal complex [Cu(pdto)(H2O)]2+ did not present a SOD-like catalytic mechanism. However, the results presented here support the idea that it is possible to study by electrochemical techniques the reaction between superoxide and a compound of interest, in order to associate the biological activity with its ability to participate in a SOD-like catalytic mechanism.
Experimental
Chemicals
All chemicals were purchased from Aldrich or Acros Organics and were used as received. DMSO 99.7% Extra Dry over molecular sieve (Acros Organics) was used in all experiments.
Synthesis
The ligand 1,8-Bis(2-pyridyl)-3,6-dithioctane (pdto) was prepared by the method described by Goodwin and Lions. Yield 70% [29]. Elemental analysis: Calcd. for C16H20N2S2: %C, 63.1; %H, 6.6; %N, 9.2; %S, 20.1. Found: %C, 63.1; %H, 6.2; %N, 9.7; %S, 20.5.
The compound Aquo 1,8-bis(2-pyridyl)-3,6-dithiaoctane copper (II) hexaflurophosphate, [Cu(pdto)(H2O)](PF6)2 was obtained by mixing 0.304 g (1.0 mmol) of pdto and 0.294 g of Cu(NO3)2 ·2.5 H2O (1.0 mmol) in 50 mL of anhydrous methanol. The solution was stirred and a deep blue color was observed. Two equivalents of ammonium hexafluorophosphate (0.0088 g) were added to the reaction mixtures and a blue precipitate was obtained. Elemental analysis: Calcd. for CuC16H21N2S2OP2F12; %C, 28.4; %H, 3.1; %N, 4.2; %S, 9.5. Found: %C, 28.1; %H, 3.0; %N, 4.7; %S, 9.3.
UV-Visible spectroscopy
UV-Vis spectra were obtained with a Thermo Evolution Array spectrophotometer in a range from 200 to 1000 nm. All measurements were performed in a quartz cell with optical path of 1cm. The measurements were performed in anhydrous DMSO.
Electrochemical experiments
The electrochemical experiments were performed using a potentiostat/galvanostat VoltaLab, PGZ 301, Radiometer Copenhagen, controlled with a PC. DMSO solutions containing 0.1 M TBABF4 were used as supporting electrolyte. All electrochemical measurements were performed using a typical three-electrode arrangement. A commercial platinum disk electrode (diameter = 2 mm) was used as working electrode, a platinum wire was used as auxiliary electrode and a silver wire was employed as pseudo-reference electrode in all experiments. Prior to using the working electrode, it was polished with diamond powder, rinsed with distilled water and sonicated for 2 minutes. Before each measurement the electrode is polished with α-alumina (0.6 micron), rinsed with distilled water and dried. The solutions were also bubbled with nitrogen before each experiment. All potential values are reported vs the couple Fc/Fc+, according to the IUPAC convention [30]. Under the above conditions, cyclic voltammetry experiments were carried out in cathodic and anodic direction starting from open circuit potential (Ei=0).
Preparation of superoxide solutions in DMSO
7.4 mg (10mmol) of KO2 and 26.2 mg (20 mmol) of 18-crown-6 ether were added to 10 mL of DMSO containing 0.1 M TBABF4, the mixture was stirred for 1 hour, until a pale yellow solution was obtained [31]. This solution was used for electrochemical measurements.
Acknowledgement
The authors thank CONACyT (130500) for financial support; M. M-E thanks CONACyT for a scholarship
References
1. McCord, J.M.; Fridovich, I. Free Rad. Biol. Med. 1988, 5, 363-369. [ Links ]
2. Batinić-Haberle, I.; Rebouças, J. S.; Spasojević, I. Antiox. Redox Sign. 2010, 13, 877-918. [ Links ]
3. Dhar, S.K.; St Clair, D.K. Free Radic. Biol. Med. 2012, 52, 2209-2222. [ Links ]
4. Buettner G.R. Anticancer Agents. Med. Chem. 2011, 11, 341-346. [ Links ]
5. Pani, G; Colavitti, R.; Bedogni, B.; Fusco, S.; Ferraro, D.; Borrello, S.; Galeotti, T. Curr Med Chem. 2004, 11, 1299-308. [ Links ]
6. Oberley, W.L.; Buettner, R.G.; Cancer Res. 1979, 39, 1141-1149. [ Links ]
7. Azad, M.; Afzaal, M.O.; Brien, P. Chem. Rev. 2010, 110, 4417-4446. [ Links ]
8. Benyahia, B.; Campana, F.; Perderau, B; Gez, E.; Fourquet, A.; Magdelenat, H. The Breast, 1996, 5, 75-81. [ Links ]
9. Vecchio, G.; Lanza, V. J. Chem. Educ. 2009, 86, 1419-1421. [ Links ]
10. Carceller, V.J.A, Universidad de Lleida,Tesis, 2007. [ Links ]
11. Gerasimchuk, N; Wang, Z; Sessler, J.L.; Magda, D.J. J. Am. Chem. Soc. 2005, 110-136. [ Links ]
12. Salvemini, D.; Riley, D. P. ; Cuzzocrea, S. Nat. Rev. Drug Discov. 2002, 1, 367-374. [ Links ]
13. Cabello, C.M.; Bair, W.B. 3rd; Wondrak G.T. Curr. Opin. Investig. Drugs, 2007, 8, 1022-1037. [ Links ]
14. Urquiola, C. ; Gambino, D.; Cabrera, M. ; Lavaggi, M.L.; Cerecetto, H.; González, M.; Cerain, A.L.; Monge, A.; Costa-Filho, A.J.; Torre, M.H. J. Inorg. Biochem., 2008, 102, 119-126. [ Links ]
15. Jitsukawa, K.; Harata, M.; Arii, H.; Sakurai, H.; Masuda, H. Inorg. Chim. Acta, 2001, 324, 108-116. [ Links ]
16. Mahadevan, S.; Palaniandavar, M. Inorg. Chim. Acta, 1997,254, 291-302. [ Links ]
17. Thompson, M.; Whelan, J.; Zemon, D.J.; Bosnich, B. ; Solomon, E.I., Gray, H.B. J. Am.Chem. Soc. 1979, 101, 2482-2483. [ Links ]
18. Sakaguchi, U. ; Addison, A.W. J. Chem. Soc., Dalton Trans. 1979, 600-608. [ Links ]
19. Mahadevan, S.; Palaniandavar, M. Inorg. Chim. Acta, 1997,254, 291-302 [ Links ]
20. Rodríguez-Torres, D.; García-Ramos, J.C. ; Manríquez, J. ; Moreno-Esparza, R.; Altamirano Lozano, M.; González, I. ; Gracia-Mora, I.; Ruiz-Azuara, L. Antaño Lopez, R. Ortiz-Frade, L. Polyhedron 2009, 28, 1186-1190. [ Links ]
21. Kuthan, H.; Haussmann, H.-J. ; Werringloer, J. Biochem. J. 1986, 237, 175-180. [ Links ]
22. Beauchamp, C.; Fridovich, I. Anal. Biochem., 1971, 44, 276-287. [ Links ]
23. Hyland, K. ; Voisin, E. ; Banoun, H.; Auclair, C. Anal. Biochem., 1983, 135, 280-287. [ Links ]
24. Moscone, D.; Mascini, M. Anal. Chim. Acta. 1988, 211, 195-204 [ Links ]
25. Rigo, A.; Viglino, P.; Rotilio, G. Anal. Biochem. 1975, 68, 1-8 [ Links ]
26. Lever, A. B. P., Inorganic Electronic Spectroscopy, 2a Ed. Elsevier, 1984. [ Links ]
27. Bard, A. J.; Faulkner, L. R. Electrochemical Methods, Fundamentals and Applications; Ed. John Wiley and Sons, 1980. [ Links ]
28. Sawyer, D.T.; Sobkowiak A, L. J.; Roberts J. Electrochemistry for chemists. 2a Ed., New York, 1995. [ Links ]
29. Goodwin, H.A; Lions F. J. Am. Chem. Soc. 1960, 82, 5013-5023. [ Links ]
30. Gritzner, G.; Küta, J. Pure Appl. Chem., 1984, 4, 461-466 [ Links ]
31. Valeriy, S.V; Roth, J.P. J. Am. Chem. Soc. 2006, 128, 3683-3695. [ Links ]














