Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.57 no.2 Ciudad de México abr./jun. 2013
Article
Smart pH/temperature Sensitive Hydrogels with Tailored Transition Temperature
Rodolfo Salgado-Rodríguez,1 Ángel Licea-Claverie,1* Arturo Zizumbo-López,1 and Karl-Friedrich Arndt2
1 Instituto Tecnológico de Tijuana, Centro de Graduados e Investigación, A.P. 1166, 22000 Tijuana, B.C., México. aliceac@ tectijuana.mx
2 Dresden University of Technology, Physical Chemistry of Polymers, D-01062 Dresden, Germany.
Received September 26, 2012
Accepted April 22, 2013
Abstract
Dual pH- and temperature-sensitive hydrogels have been prepared based on N-isopropylacrylamide (NIPAAm) and either potassium 5-methacryloyloxy-pentanoate (M4K) or potassium11-methacryloyloxy-undecanoate (M10K) as comonomers. The gels containing M10K (longer methylene chain) showed a slightly lower swelling capacity than the gels containing M4K. Hydrogels were produced with specific phase transition temperature (Tcr) in the range from 12 to 76 oC for NIPAAm-C4 hydrogels (containing M4K) and from 16 to 36 oC for NIPAAmC10 hydrogels (containing M10K) by controlling: the type and comonomer content, and the pH of water. The type of transition changes from discontinuous (below the critical pH of the acid comonomer) to continuous (above de critical pH of the acid comonomer). The title hydrogel materials are promising candidates for biomedical applications.
Key words: Smart polymers, polymer gels, N-isopropylacrylamide, hydrogels, swelling.
Resumen
Se prepararon hidrogeles sensibles tanto a la temperatura como al pH en base a N-isopropilacrilamida (NIPAAm) y 5-metacriloiloxipentanoato de potasio (M4K) o 11-metacriloiloxi-undecanoato de potasio (M10K) como comonómeros. Los geles que contienen M10K (cadenas de metileno más largas) mostraron una menor capacidad de hinchamiento que los geles con M4K. Se obtuvieron hidrogeles con temperaturas de transición de fase (Tcr) desde 12 hasta 76 °C para NIPAAm-C4 (conteniendo M4K) y de 16 a 36 °C para NIPAAm-C10 (conteniendo M10K) al controlar el tipo y cantidad de comonómero y el pH del agua. La transición de fase cambió de discontinua (abajo del pH crítico del comonómero ácido), a continua (arriba del pH crítico del comonómero ácido). Los materiales preparados son candidatos prometedores para aplicaciones biomédicas.
Palabras clave: Polímeros inteligentes, geles poliméricos, N-isopropilacrilamida, hidrogeles, hinchamiento.
Introduction
During the last twenty years, sensitive polymeric materials to external stimuli have received much attention because of their scientific and technological importance [1], they can undergo continuous or discontinuous changes in swelling response such as changes in temperature, pH, solvent, ionic strength, electric and magnetic fields or light. Among these systems, pH- or/and temperature-responsive hydrogels have been extensively studied [2-26]. This type of sensitive hydrogels offer a great potential in various applications, e.g., in the biomedical field for controlled drug release [2, 5, 19, 23, 27]. It is well known that poly(N-isopropylacrylamide) (PNIPAAm) is a temperature-sensitive polymer showing a lower critical solution temperature (LCST) or phase transition from its expanded to contracted state when is heated in water at about 30-32 oC [28]. This thermosensitive polymer has been crosslinked by different methods to form hydrogels. Like linear polymers of PNIPAAm, hydrogels exhibit a critical temperature (Tcr ≈ 32oC) above which the hydrogel undergoes a reversible volume phase transition [29-31]. This phenomenon is characterized by a large shrinkage on heating accompanied by expelling of water out of the crosslinked structure. On the contracted state, the PNIPAAm chains show more interaction with its own chains (intra- and inter-molecular interaction), so that, the material switch from a hydrophilic to a more hydrophobic state. Copolymeric hydrogels of NIPAAm have been prepared in order to alter the hydrophobicity or hydrophilicity of their chains and hence the Tcr of PNIPAAm [2-22, 24, 32].
Hydrogels sensitive to pH have been prepared when NIPPAm is copolymerized with comonomers containing acidic or basic functional groups [33-35]. The presence of ionizable groups implies a dependency of the swelling degree with external conditions, e.g., pH, ionic strength, or specific counterions since swelling in the case of hydrogels containing ionizable groups, is mainly due to the electrostatic repulsions of the same charges present in the polymer network. It has been recognized that the introduction of hydrophobic groups also influences swelling by the formation of intra- or intermolecular associations of the hydrophobic moieties resulting in a decrease in the swelling degree [36]. Previously we have shown that the introduction of hydrophobic groups as spacers between ionizable acid groups in the main chain of polymers results in a large change in the acidity constant (pKa) of this groups [37, 38]. This change in pKa results in changes in the pH triggered swelling to collapsing transition in analogous polymer networks [39, 40]. The interplay between temperature- and pH-sensitivity has been recognized recently in the case of linear polymers [41-43]. The LCST of PNIPAAm (32 oC) was shifted by changes in pH to higher or lower values, depending on the pKa's of comonomers used [42]. Dual temperature- and pH-sensitive hydrogels are being developed and tested recently for drug delivery [44] and enzyme immobilization [45], among others [46]. In this work, we report on the preparation of "smart" pH-controlled temperature sensitive copolymeric hydrogels based on NIPAAm-M4K, and NIPAAm-M10K (Fig. 1). The hydrogels were synthesized by free radical polymerization in aqueous solution with N,N'-methylenbisacrylamide (BIS) as crosslinker. The resulting hydrogels are dual pH- and temperature-sensitive materials.

We focus this study in the influence of hydrophobic spacer length, e.g. methylene chains of 4 and 10 methylene units as comonomer content and effect of pH into the Tcr behaviour of hydrogels. This hydrogels have a great potential of application in the biomedical field.
Results and Discussion
Composition of Hydrogels and Thermal Behavior
Table 1 contains the nomenclature used for the hydrogels discussed in this contribution and the molar ratios used in their preparation. All obtained gels were transparent indicating no phase-separation during synthesis, this is important since the crosslinking reaction was strong exothermic and a phase-separation could introduce heterogeneities into the polymer network structure. For all prepared hydrogels, a full incorporation of BIS was assumed, since the majority of the content is NIPAAm, a monomer known to readily polymerize with BIS; in this way the composition of the copolymeric hydrogels could be calculated from the N%/C% results. The calculated composition (Table 2) is very close to the composition used in the preparation recipe, except for C405, where a lower content of M4K was obtained (2 mol%). It is well known that the crosslinker makes a network polymer more rigid than the corresponding linear polymer; this should lead to an increase of the glass-transition temperature (Tg) as compared with a non-crosslinked polymer system of the same composition. However, if the crosslinking degree is very small this expected effect is not observed [3, 47]. As Table 2 shows, Tg of GNIPAAm network is 151 oC, a value much higher than the reported for linear polymer (137 oC) (42), indicating a more rigid structure. As expected for copolymer networks, the Tg for GNIPAAm-C4 decreases with increasing comonomer content from 151°C for 05 % of C4 to 133°C for 20% of C4, this is due to the incorporation of more flexible C-C bonds of the substituents in the comonomers structure; however this decrease is small as compared with the decrease in Tg of linear copolymers [42].
This is because the crosslinker makes the material in general more rigid. The same can be observed in the copolymeric NIP-PAAm-C10 hydrogels, where the Tg decreases due to the increment of the flexibility of the substituent of the comonomeric unit from 140°C for a 5% of C10 to133°C for 20% of C10. The contribution of the flexibility for C-C bond from the substituent can be easily observed comparing a GNIPAAm95-C1005 hydrogel (5% of C10) with Tg of 140°C which is lower than the GNIPAAm95C405 with the same composition (5% of C4) with a Tg of 147°C.
Swelling Experiments
Swelling behavior for temperature sensitive hydrogels with relatively low crosslinking density has been reported to be almost independent on the chemical crosslinker amount [3, 47]. However, equilibrium swelling capacity for the hydrogels decreases with an increase in the crosslinker concentration. In fact, a high crosslinker content makes the gel network dense and less flexible and expandable, which restricts the diffusion of water molecules into the hydrogel network frame and therefore lowers its swelling capacity [12, 14, 16-18, 24, 30, 34, 36, 47]. In our case the concentration of crosslinker in the copolymeric hydrogels and homopolymeric NIPAAm hydrogel was not changed so that the observed differences in swelling behaviour can be attributed to the comonomer effect into the swelling behavior.
The swelling ratio (r = DpH/DpH0) at 25°C was studied as a function of pH in buffer solutions for homopolymeric GC4 and GC10 hydrogels furthermore, the swelling ratio as a function of temperature (r = DT/DT0) at fixed pH-values was studied for GNIPAAm in water and for GNIPAAm-C4 and GNIPAAm-C10 copolymeric hydrogels in buffer solutions. Hydrogels without NIPAAm (GC4 and GC10) were not analyzed regarding the temperature because these hydrogels lack of a critical temperature phenomenon in pure water.
The swelling ratio at room temperature for hydrogels GC4 and GC10 in solutions with different pH values is plotted in Figure 2. For the ratio r = DpH/DpH0, DpH0 is used as reference value at pH = 3. Also the derivative of r with respect to pH was plotted. The maximum of this curve was taken as the critical pH (by definition the pH at which the larger change of volume is observed). This analysis was very important in order to understand the effect of the pH into the M4K and M10K units in the corresponding copolymeric hydrogels.

Results show a sharp peak for the GC4 hydrogel with a maximum signaling a critical value of pH of 5.96, while the peak for the GC10 hydrogel is wider with a maximum at a pH value of 7.7. These values correspond well with the acidity constants reported for linear polymers prepared with these monomers [42] with a pKa= 6.1 for C4 and pKa = 7.3 for C10. Also, GC4 shows a larger change in volume within a narrow pH-range (between 5 and 6), this is an indicator of a discontinuous transition with a significant absorption of water by varying slightly the pH.
On the other hand, GC10 shows a smaller and gradual change in the volume within a broad pH range (between 5 and 10), this fact is a typical feature of continuous transition with pH attributed to the intra- and inter-molecular interactions given by the stronger hydrophobic character of GC10 as compared with GC4.
The swelling behavior in water for GNIPAAm-C4 and GNIPAAm-C10 copolymeric hydrogels is shown in Figure 3 for different compositions as a function of temperature.
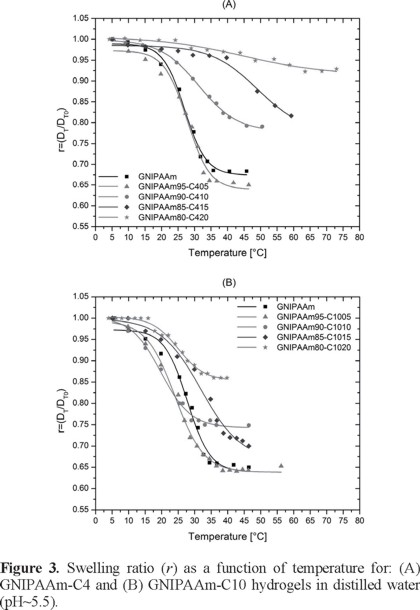
As reported for the GPNIPAAm hydrogel, the copolymeric hydrogels were sensitive to temperature changes of the swelling medium. In fact, in the case of GNIPAAm-C4 hydrogels (Fig. 3, A), temperature rise causes a decrease on the diameter of gel samples and hence a decrease on the amount of aqueous solution retained by those gels. The transition magnitude of the gel shrinkage is smaller and wider in a temperature range when the content of comonomer C4 increases as consequence of the more hydrophobic C4 contribution, and the transition almost disappears with a content of C4 totaling 20%. The same effect occurs by increasing the C10 content for GNIPAAm-C10 hydrogels (Fig. 3, B). However, the transition temperature is sharper for GNIPAAm-C10 hydrogels than for GNIPAAm-C4 hydrogels due to the more hydrophobic C10 than C4 comonomer. In other words, the intra- and inter-molecular interactions are preferred when the comonomer is more hydrophobic, and the water is expelled from the hydrogel in a narrow temperature range.
When the solution pH is considered as a variable (Fig. 4, for GNIPAAm-C4), the range of temperatures at which the hydrogel shrinks, is changed. Furthermore, the swelling capacity of the hydrogel is also affected by pH, since the acid groups of the comonomers are weak electrolytes responding to different pH-values in the environment with differences in ionization degree. So that, the effect of pH of the medium on transition temperature behavior for all these hydrogels exhibit a gradual shrinkage, which occurs in a wide range of temperatures. The sharper transition is observed at acidic pH values, where the hydrophobic interactions are favored due to the acid nature of the comonomer C4. At acidic pH values the comonomers are already in the acid state, in other words the potassium salt was changed due to the protonation of the comonomer. In fact, when the pH is increased, the ionization degree of the acid comonomers increases resulting in a wider temperature transition range for the hydrogel shrinkage.
In the plots the remarkable effect caused by increasing the comonomer content can be observed. For GNIPAAm-C4 hydrogels the increase on the amount of C4 caused a separation of the deswelling curves between pH = 5 and pH = 6 (Fig. 4), this range being exactly the critical pH range for the GC4 hydrogel (pH 5.96, Fig. 2). The separation of the shrinkage curves is greater the higher the content on C4 units in the hydrogel.
The swelling behavior in buffer solutions for GNIPAAm-C10 hydrogels with different compositions is reported in Figure 5. For GNIPAAm-C10 hydrogels no bigger separation of the shrinking curves occurs at a certain pH-value, the separation in the shrinking curves with pH is gradual in agreement with the behaviour shown for the non-thermosensitive GC10 hydrogel with pH, where the change of swelling ratio (r) occurs in a continuous form. However, an interesting feature is that the biggest contraction for those kind of hydrogels (C10-series) is always shown at pH 8; exactly the average pH for the shrinkage to swelling pH-transition (pH 7.7, Figure 2).
The critical temperatures of the gels were calculated from the plots of swelling ratio (r) versus temperature. The first derivative of dr/dT was used as indicator of the maximum shrinkage change with temperature. The temperature where this change shows a maximum value was taken as the critical temperature (Tcr). Table 3 shows the results for Tcr calculated this way. These values are plotted in Figure 6 as a function of pH with a statistical fitting. If we analyze first the GNIPAAm-C4 hydrogels (Figure 6, A), we can see that all curves intercept at a given pH value.
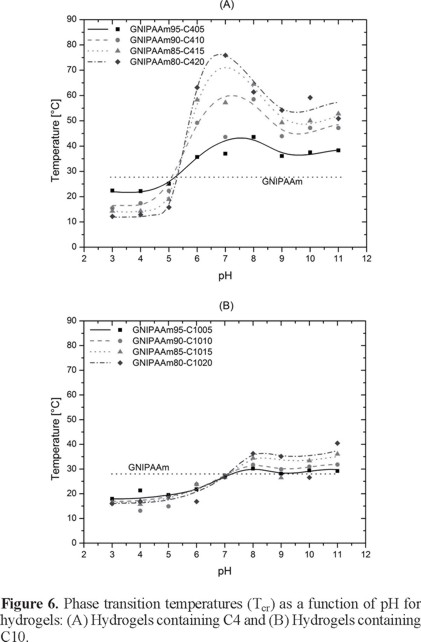
This pH value (5.5) corresponds more or less with the critical pH value of GC4 hydrogels of 5.96 and with the critical pH for C4 homopolymer (pH = 5.9). Below this point we can observe, a decrease of the transition temperature when the comonomer content is increased. This is attributed to the stronger hydrophobic effect at low pH values where the acid comonomer is not ionized. After that pH value, the ionization effect is more important, and the transition temperature increases with increasing comonomer content as a consequence of the ionic contribution of the comonomer C4 in the ionized state. In this state, increased content on ionized groups, results in higher energy needed to overcome the increased hydrogen bonding sites to water molecules to trigger the shrinkage of the hydrogel, therefore increasing the transition temperature. However, the transition point reaches a maximum value, where the effects of ionic interactions are shielded by the salt content of the buffer solutions. After that, the transition temperature decreases with increasing pH value until the pH is high enough for hydrolysis of some side groups with a slightly increase in the transition temperature for values higher than pH 10. The same effect is observed if we analyze the GNIPAAm-C10 hydrogels (Figure 6, B). However the change in transition temperatures is smaller for GNIPAAm-C10 hydrogels than for GNIPAAm-C4 hydrogels, this is attributed to the stronger hydrophobic nature of C10 as compared with C4 comonomer, due to higher number of methylene units in the spacer group. Below of a critical pH value (pH = 7.0, Figure 6, B), the reduction of transition temperature for these copolymeric hydrogels is small when the content on C10 is increased. Over this critical pH, the transition temperature increases clearly with increasing content on C10 resulting from the increased ionic effect from the acid comonomer at pH values higher than 7.
In general, copolymeric hydrogels show a discontinuous phase-transition temperature at pH values lower than the critical pH of the comonomer. On the other side, the change in transition temperature is bigger at pH values higher than the critical pH of the comonomer. With these results, copolymeric hydrogels with a balance between desired temperature sensitivity and swelling capacity that can be used for a specific application, can be designed and prepared.
Conclusion
NIPAAm-copolymeric hydrogels with dual sensitivity to pH and temperature were prepared successfully. The use of monomers in their potassium salt form, M4K and M10K, besides of NIPAAm, brings great advantages on the preparation method: polymerization at room temperature, use of water instead of organic solvents and their compatibility with biological systems (aqueous) important for biomedical applications.
The sensitivity to pH is given by the carboxylic acid units in the comonomer which are weak electrolytes, and therefore their dissociation degree is closely related to the pH value of the medium.
The prepared hydrogels showed a wide range of critical temperatures (Tcr), from 12 to 76 oC. However, critical temperatures with discontinuous transitions are shown only for low pH values where the hydrophobic effects are more significant than the ionic effects since the acid comonomers are not or only slightly ionized. Furthermore, continuous temperature transitions are shown for high pH values where the ionic interactions are predominant.
For copolymeric hydrogels containing M4K, a critical pH was observed at about pH = 5.5, whereas for those containing M10K the critical pH was observed at about pH = 7. At pH values lower than the critical pH, the transition temperature decreases with increasing commoner content while at pH values higher than the critical pH, the transition temperature increases with increasing commoner content.
A general conclusion is that it is possible to design a hydrogel with a specific transition temperature and swelling degree as a function of the type and content of comonomer (number of methylenes as spacer groups), for an specific pH of the medium in which they will be used. We suggest that several of the prepared materials are excellent candidates for the controlled delivery of drugs by changes in pH and/or temperature.
Experimental
Materials
N-isopropylacrylamide (NIPAAm), N,N'-methylenebisacrylamide (BIS), ammonium persulfate (APS), Hexane (ACS grade) and N,N,N',N'-tetramethylethylenediamine (TEMED) were purchased from Aldrich Chemical Co. Potassium Hydroxide, methanol (anhydrous) and diethylether (anhydrous) were purchased from FERMONT (Monterrey, México). NIPAAm was purified by recrystallization from hexane while the other chemicals were used as received. Potassium 5-metacryloyloxy-pentanoate (M4K) and Potassium 11-metacryloyloxy-undecanoato (M10K) monomers were synthesized using chemicals and solvents as described below. For all hydrogel preparations and swelling experiments, twice distilled water was used. For ionic strength (0.1 M) adjust, buffer solutions were prepared using acetic acid, sodium acetate, sodium chloride, HCl, boric acid, sodium tetraborate Tris(hydr oxymethyl)aminomethane, and NaOH.
Synthesis of Monomers as Potassium Salts of Methacrylic Acid Derivatives
Monomers with a terminal carboxylic acid group, 5-methacryloyloxyvaleric acid (MOD4) and 11-methacryloyloxyundecanoic acid (MOD10), were synthesized as described previously by our group [42]. These monomers were converted into their respective potassium salts, by addition of potassium hydroxide (1:1 molar ratio) in a methanolic solution. Using this methodology, we prepared the following monomers: Potassium 5-methacryloyloxy-pentanoate (M4K) with n=4 and Potassium 11-methacryloyloxyundecanoate (M10K), with n = 10. The detailed procedure is described only for the preparation of M4K: MOD4 (10 g, 0.054 mol) was dissolved in methanol (10 mL). Separately, KOH (3.0134 g, 0.054 mol) was dissolved in methanol (10 mL). Both solutions were mixed slowly and left stirring for 6 h. Ethyl ether (20 mL) was added to precipitate the product: potassium 5-methacryloyloxi-pentanoate (M4K). The product was then filtered-off, washed with ethyl ether (3 × 20 mL) and finally dried at 40 oC for 48 h. The product was obtained as a white solid (9.9 g, 0.044 mol, 82% yield).
Synthesis of GNIPAAm-C4 and GNIPAAm-C10 Hydrogels
The hydrogels were prepared in twice distilled water, using N,N'-methylenbisacrylamide (BIS) as the crosslinking agent, ammonium persulfate (APS) as the initiator and N,N,N',N'-tetramethylethylenediamine (TEMED) as accelerator. APS and TEMED were used as 0.15 M solutions in twice distilled water. Hydrogels in different compositions were prepared: 3 homopolymeric hydrogels (GNIPAAm, GC4 and GC10) and 8 copolymeric hydrogels (GNIPAAm-C4 or GNIPAAm-C10) (Table 1). In all cases the following concentrations were used: TEMED 1 mol% and APS 1 mol% taking as a basis the total number of monomer moles; BIS 9 mol% for GC4 and GC10 homopolymeric hydrogels and BIS 3 mol% for GNIPAAm homopolymeric and GNIPAAm-C4, GNIPAAm-C10 copolymeric hydrogels.
A total monomer concentration of 15.6 mmol in 10 mL of water was used for copolymeric (GNIPAAm-C4 and GNIPAAmC10) and homopolymeric GNIPAAm hydrogels; while a monomer concentration of 1.765 g in 10 mL of water was used for the other two homopolymeric GC4 and GC10 hydrogels. Copolymeric hydrogels were prepared by varying the molar content of the comonomer (M4K or M10K) feed: 5, 10, 15 and 20 mol%.
Monomers, BIS and twice distilled water were added to a flask and stirred at room temperature about 30 min, until the mixture had uniform consistency. A solution of APS 0.15 M was then added. The flask was sealed and argon gas was bubbled into the solution for 30 min. The solution was then cooled in an ice bath for 5 min. After this, TEMED solution was added to the flask and immediately the solution poured into the container to allow hydrogel formation.
All hydrogels were prepared as cylindrical rods (using capillary tubes as molds). Capillary tubes (100 × 1.0 mm Ø) open in both sides and test tubes (100 × 16 mm Ø) were used. 25 capillary tubes were placed in a test tube inside an ice bath. The solution was added slowly to fill up the capillary tubes. Then these were shaken until no bubbles were observed inside them. The system was sealed with ParafilmTM and left in the fridge (≈ 4 oC) for 24 h, to make sure that all the comonomers were fully incorporated (even if the formation of the gel was visible in the first two hours). The prepared hydrogels were extracted from capillary tubes and placed afterwards in 40 mL vials with deionized water for 7 days, changing the water daily to remove the unreacted reagents.
Characterization
Swelling Measurements
Cylindrical hydrogel rods were placed in 10 mL vials containing either twice distilled water or buffer solutions (pH = 3-11). The Buffer solutions were changed daily from the vials until the pH was constant (3 to 4 days); after this fresh Buffer (same pH) was added and the hydrogel left for a week to reach equilibrium. After this procedure the hydrogels were ready for swelling studies at different temperatures. These studies were carried out by measuring the change in the diameter with the temperature for ten specimens from the same sample and the average measurement were used for all analysis.
Imaging Measurements
Measurements of diameter were performed with the IMAGING SYSTEM Analysis 2.11 software, adapted to a JVC camera model TK-C138OE. For cylinders of GNIPAAm hydrogels, the swelling ratio was studied only in twice distilled water (no Buffers); while for cylinders of GC4 and GC10 hydrogel, the swelling ratio at a constant temperature of 25 oC was studied in buffer solutions of different pH's ranging (3 to 11). A temperature response study was carried out for each copolymeric hydrogel in a fixed pH buffer solution (3 to 11), from 5 °C to 75°C or until a constant diameter of the discs with increments de 5 °C/10 min.
Each cylindrical hydrogel was placed in the respective solution inside a container connected to a recirculating bath so the temperature could be controlled and fixed. The experiments began with a temperature of 5 oC, leaving this temperature for 20 min. After this time, the container temperature was recorded, the image in the computer frozen and the gel diameter estimated as the average of ten measurements at different spots of the hydrogel-cylinder image on screen. The temperature was risen 5 oC and the system left at this new temperature for 10 min. After this time, the measurements were performed (over ten spots and averaged) and the temperature increased again for another 5 oC. This procedure was repeated increasing the temperature in 5 oC steps until the diameter of the gel was maintained constant or the temperature reached a final value of 70-75 oC. The swelling ratio (r = DT/DT0; DT the diameter at a given temperature and DT0 being the diameter at 5 oC) was plotted versus temperature.
Thermal Analysis
The glass transition temperature (Tg) of purified and dried hydrogels (vacuum oven at 40 oC for a week, and 72 h before analysis) was obtained by using a Mettler DSC-30 calorimeter at a heating rate of 10 K/min. Samples were analyzed in closed aluminum cups.
Elemental Analysis
The chemical composition of dry gels was obtained by C, H and N elemental analysis using a Carlo Erba CHNS-O EA 1108 equipment. The monomer content was calculated by using the ratio %C/%N to eliminate the effect of residual humidity present in the samples. For the copolymers, it was assumed that BIS-crosslinker was incorporated with the same percentage as used in the feed (3 mol% on the basis of total monomers) because it reacts with two vinyl bonds in the network structure.
Acknowledgements
This work received financial support from CONACYT-Mexico (Project Nr. 28022-U) and from the Volkswagen Foundation-Germany (Project Nr. I/76 065). R. Salgado-Rodriguez thanks DAAD-Germany and CONACYT-Mexico for financing his PHD-Sandwich Program. We thank specially for valuable technical support I. Poitz (DSC).
References
1. Kumar, A.: Srivastava, A.; Galaev, I.Y.; Mattiasson, B. Prog. Polym. Sci. 2007, 32, 1205-1237. [ Links ]
2. Sousa, R.G.; Prior-Cabanillas, A.; Quijada-Garrido, I.; Barrales-Rienda, J.M. J. Controlled Rel. 2005, 102, 595-606. [ Links ]
3. Prior-Cabanillas, A.; Quijada-Garrido, I.; Frutos, G.; Barrales-Rienda, J.M. Polymer 2005, 46, 685-693. [ Links ]
4. Quijada-Garrido, I.; Prior-Cabanillas, A.; Garrido, L.; Barrales-Rienda, J.M. Macromolecules 2005, 38, 7434-7442. [ Links ]
5. Diez-Pena, E.; Frutos, P.; Frutos, G.; Quijada-Garrido, I.; Barrales-Rienda, J.M. AAPS Pharm. Sci. Tech. 2004, 5, 1-8. [ Links ]
6. Diez-Pena, E.; Quijada-Garrido, I.; Frutos, P.; Barrales-Rienda, J.M. Macromolecules 2002, 35, 2667-2675. [ Links ]
7. Zhang J.; Chu, L.Y.; Li, Y.K.; Lee, Y.M. Polymer 2007, 48, 1718-1728. [ Links ]
8. Tao, Y.; Zhao, J.X.; Wu, C.X. J. Appl. Polym. Sci. 2006, 101, 323-330. [ Links ]
9. Xue, W.; Champ, S.; Huglin, M.B.; Jones, T.G.J. Eur. Polym. J. 2004, 40, 467-476. [ Links ]
10. Tian, Q.; Zhao, X.; Tang, X.Z.; Zhang, Y.X. J. Appl. Polym. Sci. 2003, 87, 2406-2413. [ Links ]
11. Zhang, X.Z.; Yang, Y.Y.; Wang, F.J.; Chung, T.S. Langmuir 2002, 18, 2013-2018. [ Links ]
12. Mohan, Y.M.; Joseph, D.K.; Geckeler, K.E. J. Appl. Polym. Sci. 2007, 103, 3423-3430. [ Links ]
13. Mohan, Y.M.; Geckeler, K.E. React. Funct. Polym. 2007, 67, 144-155. [ Links ]
14. Mohan, Y.M.; Premkumar, T.; Joseph, D.K.; Geckeler, K.E. React. Funct. Polym. 2007, 67, 844-858. [ Links ]
15. Hirashima, Y.; Sato, H.; Suzuki, A. Macromolecules 2005, 38, 9280-9286. [ Links ]
16. Chen, H.; Hsieh, Y.L. J. Polym. Sci. A: Polym. Chem. 2004, 42, 3293-3301. [ Links ]
17. El-Hamshary, H. Eur. Polym. J. 2007, 43, 4830-4838. [ Links ]
18. Krusic, M.K.; Filipovic, J. Polymer 2006, 47, 148-155. [ Links ]
19. Katime, I.; Valderruten, N.; Quintana, J.R. Polym. Int. 2001, 50, 869-874. [ Links ]
20. Weiss-Malik, R.A.; Solis, F.J.; Vernon, B.L. J. Appl. Polym. Sci. 2004, 94, 2110-2116. [ Links ]
21. Bai, G.; Suzuki, A. Eur. Phys. J., E 2004, 14, 107-113. [ Links ]
22. Kuckling, D.; Richter, A.; Arndt, K.F. Macromol. Mater. Eng. 2003, 288, 144-151. [ Links ]
23. Lee, W.F.; Lin, Y.H. J. Appl. Polym. Sci. 2006, 102, 5490-5499. [ Links ]
24. Katime, I.; Quintana, J.R.; Valderruten, N.E.; Cesteros, L.C. Macromol. Chem. Phys. 2006, 207, 2121-2127. [ Links ]
25. Caykara, T.; Kiper, S.; Demirel, G.; Demirci, S.; Cakanyildirim, C. Polym. Int. 2007, 56, 275-282. [ Links ]
26. Zhang, K.; Luo, Y.; Li, Z. Soft Materials 2007, 5, 183-195. [ Links ]
27. Guo, B.L.; Gao, Q.Y. Carbohydr. Res. 2007, 342, 2416-2422. [ Links ]
28. Boutris, C.; Chatzi, E.G. ; Kiparissides, C. Polymer 1997, 38, 2567-2570. [ Links ]
29. Rice, C.V. Biomacromolecules 2006, 7, 2923-2925. [ Links ]
30. Ju, X.J.L.; Chu, L.Y.; Zhu, X.L.; Hu, L.; Song, H.; Chen, W.M. Smart Mater. Struct. 2006, 15, 1767-1774. [ Links ]
31. Zhao, Q.; Sun, J.; Zhou, Q. J. Appl. Polym. Sci. 2007, 104, 4080-4087. [ Links ]
32. Ebara, M.; Aoyagi, T.; Sakai, K.; Okano, T. J. Polym. Sci. A: Polym. Chem. 2001, 39, 335-342. [ Links ]
33. Wen, S.; Stevenson, W.T.K. Colloid Polym. Sci. 1993, 271, 38-49. [ Links ]
34. Turan, E.; Caycara, T. J. Appl. Polym. Sci. 2007, 106, 2000-2007. [ Links ]
35. Kim, B.; Shin, Y. J. Appl. Polym. Sci. 2007, 105, 3656-3661. [ Links ]
36. Emileh, A.; Vasheghani-Farahani, E.; Imani, M. Eur. Polym. J. 2007, 43, 1986-1995. [ Links ]
37. Rogel-Hernández, E.; Licea-Claveríe, A.; Cornejo-Bravo, J.M.; Arndt, K.F. Des. Mon. Polym. 2001, 4, 343-356. [ Links ]
38. Licea-Claveríe, A.; Rogel-Hernández, E.; López-Sánchez, J.A.; Castillo, L.A.; Cornejo-Bravo, J.M.; Arndt, K.F. Des. Mon. Polym. 2003, 6, 67-80. [ Links ]
39. Rogel-Hernández, E.; Licea-Claveríe, A.; Cornejo-Bravo, J.M.; Arndt, K.F. Rev. Soc. Quim. Mex. 2003, 47, 251-257. [ Links ]
40. Licea-Claveríe, A.; Rogel-Hernández, E.; Salgado-Rodríguez, R.; López-Sánchez, J.A.; Castillo, L.A.; Cornejo-Bravo, J.M.; Arndt, K.F. Macromol. Symp. 2004, 207, 193-215. [ Links ]
41. Jones, M.S. Eur. Polym. J. 1999, 35, 795-801. [ Links ]
42. Salgado-Rodriguez, R.; Licea-Claverie, A.; Arndt, K.F. Eur. Polym. J. 2004, 40,1931-1946. [ Links ]
43. Yin, X.; Hoffman, A.S.; Stayton, P.S. Biomacromolecules 2006, 7, 1381-1385. [ Links ]
44. Milasinovic, N.; Milosavljevic, N.; Filipovic, J.; Knezevic-Jugovic, Z.; Krusic, M.K. Rect. Funct. Polym. 2010, 70, 807-814. [ Links ]
45. Zhao, S.P.; Cao, M.J.; Li, L.Y.; Xu, W.L. Polym. Degrad. Stabil. 2010, 95, 719-724. [ Links ]
46. Paris, R.; Quijada-Garrido, I. Eur. Polym. J. 2010, 46, 2156-2163. [ Links ]
47. Zhang, X.Z.; Wu, D.Q.; Chu, C.C. J. Polym. Sci. B: Polym. Phys. 2003, 41, 582-593. [ Links ]














