Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.57 no.2 Ciudad de México abr./jun. 2013
Article
Use of Ligand-based Iron Complexes for Phenol Degradation by Fenton Modified Process
Iván A. Reyes,1,* Francisco Patiño,1 Mizraím U. Flores,1 Jayanthi Narayanan,2 Hilda Calderón,3 and Thangarasu Pandiyan3
1 Área Académica de Ciencias de la Tierra y Materiales, Universidad Autónoma del Estado de Hidalgo, Carretera Pachuca-Tulancingo km 4.5, Pachuca Hidalgo 42184, México. ivanalejandro2001@hotmail.com
2 División de Nanotecnología, Universidad Politécnica del Valle de México, Av. Mexiquense, Tultitlán, Estado de México 54910, México.
3 Facultad de Química, Universidad Nacional Autónoma de México, Ciudad Universitaria, 04510 México, D.F., México.
Received September 14, 2012
Accepted April 19, 2013
Abstract
The efficiency of phenol degradation by the iron complexes ([Fe(TBMA)Cl3∙3H2O] and [Fe(terpy)Cl2]) is compared with that of the Fenton reaction. The results show that although the Fenton reaction efficiently oxidizes phenol at low pH's, the Fenton modified reagents (iron complexes/H2O2) effectively oxidize phenol at neutral pH. Besides, the factorial designing study is performed by considering three independent variables: (i) [Fe] (A), (ii) pH (B), and (iii) [H2O2] (C). For the Fenton reaction, the normal probability plot reveals that two factors, such as concentration of Fe2+ and interaction{[H2O2]∙pH} have considerable influence on the phenol oxidation; in the normal probability plot of the complexes, factors C (concentration of H2O2) and AC {[Fe]∙[H2O2]} have an effect on the oxidation of the phenol by [Fe(terpy)Cl2]/H2O2, while for [Fe(TBMA)Cl3∙3H2O], factors B and AC significantly influence the degradation. Of both iron complexes, [Fe(TBMA)Cl3∙3H2O]/H2O2 is an excellent oxidant, showing a good response at pH 7.0.
Key words: Modified Fenton reagent, factorial design, iron complexes, phenol oxidation.
Resumen
La eficiencia en la degradación de fenol por complejos de hierro ([Fe(TBMA)Cl3∙3H2O] y [Fe(terpy)Cl2]) es comparada con la reacción Fenton. Los resultados muestran que aunque la reacción Fenton oxida al fenol a bajo pH, la reacción Fenton modificada (complejos de hierro/H2O2) oxida efectivamente al fenol a pH neutro. También, un diseño experimental se desarrolla considerando tres variables independientes: (i) [Fe] (A), (ii) pH (B) y (iii) [H2O2] (C). Para la reacción Fenton la gráfica de probabilidad normal revela que dos factores, la concentración de Fe2+ y la interacción {[H2O2]∙pH} tienen una influencia considerable en la oxidación de fenol; en las graficas de probabilidad normal para los complejos, los factores C (concentración de H2O2) y AC {[Fe]∙[H2O2]} tienen una influencia considerable en la oxidación de fenol por [Fe(terpy)Cl2]/H2O2, mientras que para [Fe(TBMA)Cl3∙3H2O]/H2O2, los factores B y AC significativamente influencian la degradación. De ambos complejos, [Fe(TBMA)Cl3∙3H2O]/H2O2 es un excelente oxidante, mostrando una buena respuesta a pH 7.0.
Palabras clave: Reactivo Fenton modificado, diseño factorial, complejos de hierro, oxidación de fenol.
Introduction
Of the several kinds of pollutants which are present in the industrial effluents, i.e. paper, dyestuff, pharmaceutical, and agrochemical industries, phenol and its derivatives must be approached due to their high toxicity, which remains even at low concentrations in water (0.05 mg L-1), giving it a foul odor and taste [1-2]. After chlorination it forms chlorophenols, which are compounds that become toxic at concentrations higher than 2 mg L-1. It also has a high oxygen demand (2.4 mg mg-1 phenol), and it gives rise to problems coarsening the aquatic life because of the decrease in oxygen concentration [3].There are several techniques available for phenol treatment, such as separation methods: distillation, adsorption with activated carbon, and solvent-membrane extraction; and degradation methods: catalytic wet oxidation, supercritical water oxidation, ozonation, electrochemical and photocatalytic oxidation [4].
Although the photocatalytic oxidation [5] by UV light combined with photo-catalysts has been used to degrade the many toxic organic compounds in the wastewater into carbon dioxide with considerable success [6-8], it has many drawbacks because it requires expensive equipments (UV lamp/catalyst TiO2); besides, it is not easy to manage under normal conditions due to the presence of harmful UV radiation. Additionally, it is believed that these procedures generate highly toxic polychlorinated dibenzop-dioxins (PCDDs), and polychlorinated dibenzofuran (PCDFs) compounds during the UV treatment of polychlorophenols [9-11]. Thus the application of the Fenton reagent (mixture of Fe(II) and H2O2 which produces OH∙ radicals), is growing rapidly as a pretreatment method for the wastewater [10,12-14]. Furthermore, the Fenton reagent is easy to handle and environmentally benign, making this systems attractive to treat the aqueous or solid bound organic contaminants [15-18].
To improve the Fenton's process, many studies have been carried out and has been reported the applicability and some advantages of this technique possesses in the wastewater treatment [10, 12-14, 19-22]. The reaction of the Fenton reagent with phenol can be described as follows [23]:

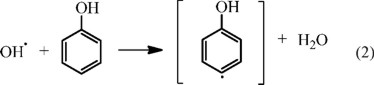
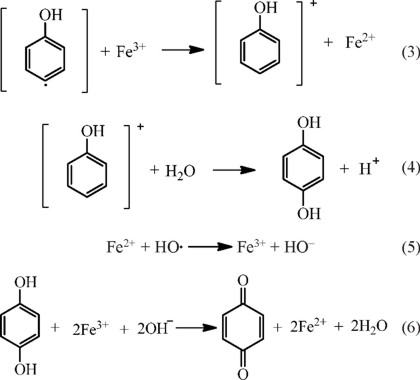
In the oxidation of phenol by Fenton reagent, the formation of benzoquinones such as ortho, para and meta isomers are established (Eq. 6) [24].
For the degradation of phenolic compounds by the Fenton process, where there are several factors that affect the efficiency of the reaction, it has been widely considered that the concentrations of Fe2+, hydrogen peroxide and pH of the reaction medium are relevant variables. It is known that OH∙ radicals are generated in the 2.0-4.0 pH range and most importantly, the rate of radical production depends on the concentration of Fe2+ and hydrogen peroxide [19, 24-27].
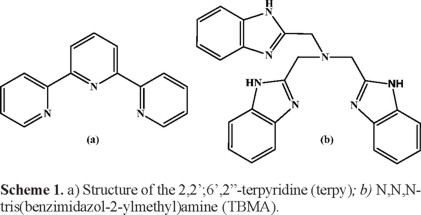
By this reason, in the present paper, a comparative study of phenol degradation between the process of conventional Fenton and the modified Fenton was carried out; iron complexes [Fe(TBMA)Cl3∙3H2O] and [Fe(terpy)Cl2] were used for the modified Fenton to see the ligand effects around metal ion in the phenol oxidation under conditions where conventional Fenton reagent has good response (acidic pH and atmospheric temperature). Both complexes were selected because 2,2';6',2"-terpyridine (terpy) is a good π-acceptor and σ-donor to stabilize Fe(II), while N,N,N-tris(benzimidazol-2-ylmethyl)amine (TBMA) (see Scheme 1) is a moderate π-acceptor and σ-donor in stabilizing Fe (II) or Fe(III). Furthermore, in order to improve the efficiency of the process, a complete statistical analysis comprising the design of experiments was carried out to assess the possibility of using the proposed complexes instead of Fe2+ as catalyst, observing the different variable effects and their interactions that directly affect the Fenton process of phenol degradation.
Results and Discussion
Experimental design: 23 factorial
Statistical models were employed to analyze the rate of the phenol degradation performed by the Fenton or the Fenton modified techniques at normal atmospheric conditions, with a view to simulate the operating conditions to obtain the maximum level of phenol degradation by employing minimum amounts of reagent. After analyzing the experimental designing studies for the oxidation by the Fenton or Fenton modified process, the degradation of phenol was experimentally performed by those processes. In order to determine the maximum and minimum factor levels, several trial experiments were carried out, indicating that the Fenton process exhibits a good response related to the phenol degradation at the levels indicated in Table 1.
In the results of the experimental design for the degradation (Table 2), the signs + or -represent the highest or lowest level of each variable.
In order to determine the effect of each factor (A, B, C, see Table 1) and its interactions, total variable combinations were used to derive the matrix design (Table 3). After analyzing the results (Table 4), it was noticed that factor A, [Fe2+] concentration, caused the highest factorial effect in the oxidation of phenol by the Fenton process, followed by pH, and then the {[Fe]2+∙[H2O2]} interaction; this means that the concentration of Fe(II) is essential, that is why at the initial stage of the reaction, where Fe(II) oxidizes to Fe(III), the reaction rate is not very high, presenting an induction period, followed by the reduction of Fe(III) to Fe(II) by hydroquinone (H2Q) (equation 9). However, for the Fenton modified process using [Fe(terpy)Cl2], factor C (hydrogen peroxide concentration) presents a factorial effect on the phenol degradation higher than the concentration of [Fe(terpy)Cl2]; the terpy group stabilizes more Fe(II) than Fe(III), this explains why it requires a higher concentration of H2O2. In the case of the process using [Fe(TBMA)Cl3∙3H2O], factor B (pH) has a predominant influence compared to other factors, since the benzimidazol group of TBMA stabilizes both Fe(II) and Fe(III), as it is a moderate π-acceptor and σ-donor. Thus, the order of effect is as follows: concentration of [Fe(TBMA)Cl3∙3H2O] > pH interaction > concentration of hydrogen peroxide. The above observations indicate that for the process with [Fe(TBMA)Cl3∙3H2O], [Fe(terpy)Cl2], increasing the factor levels from low to high maximizes the efficiency of phenol removal ( ). However, since the effect of B is negative for the reaction with [Fe(TBMA)Cl3∙3H2O], the elevation of the pH could reduce the efficiency of phenol oxidation; the same inference was found for the case of [Fe(terpy)Cl2], where factor B (pH) is negative in the designing data. In addition, the standard deviation error of the effects (MSE) was used to determine the factors that are different from zero (Table 4), as the MSE effect corresponding to zero is negligible, with a confidence interval of about 95%. Furthermore, the analysis of variance confirms that the significance of the effects is at a probability level of (1.0%).
). However, since the effect of B is negative for the reaction with [Fe(TBMA)Cl3∙3H2O], the elevation of the pH could reduce the efficiency of phenol oxidation; the same inference was found for the case of [Fe(terpy)Cl2], where factor B (pH) is negative in the designing data. In addition, the standard deviation error of the effects (MSE) was used to determine the factors that are different from zero (Table 4), as the MSE effect corresponding to zero is negligible, with a confidence interval of about 95%. Furthermore, the analysis of variance confirms that the significance of the effects is at a probability level of (1.0%).
The normal probability plot (Fig. 1) was obtained by employing all the calculated effects in MINITAB 15 Statistical Software for the phenol degradation processes; in the plot (oxidation by the Fenton reaction), it was observed that two points (A and BC) are deviated from the straight line, implying that the concentration of Fe2+ and the interaction {[H2O2]∙pH} have considerable influence in the oxidation; however, the effect of factor A is believed to be the most important in the reaction, as its deviation from the straight line is more pronounced than that of the BC. It means that the highest phenol oxidation is obtained experimentally when the concentration of Fe was higher than other factors (see Table 2). In the case of the normal probability plot of [Fe(terpy)Cl2], factors C (concentration of H2O2) and AC {[Fe]∙[H2O2]}, which are slightly deviated from the straight line, are the most important factors in the oxidation of phenol. For the case of [Fe(TBMA)Cl3∙3H2O], factors B and AC corresponding to pH and {[Fe]∙[H2O2]} have the most significant influence on the phenol degradation.

Furthermore, a statistical model was derived to predict the efficiency of the degradation of phenol (in percentage) by employing all the experimental data in equation 7.
 = β0 + β1x1 + β2x2 + β12x1x2 + β3x3 + β13x1x3 + β23x2x3 + β123x1x2x3 (7)
= β0 + β1x1 + β2x2 + β12x1x2 + β3x3 + β13x1x3 + β23x2x3 + β123x1x2x3 (7)
Where x1 = coded variable representing [Fe]; x2 = pH of the solution; x3 = [H2O2]; β = linear regression coefficients having an intercept of β0. The average of all the experimental data (Table 2) corresponds to the system response ( ). The relation between the natural variables ([Fe], pH and [H2O2]) and the coded variables is presented in equation 8:
). The relation between the natural variables ([Fe], pH and [H2O2]) and the coded variables is presented in equation 8:

Where xi = coded variable, f = initial value of the variable (xlow and xhigh are the lowest and highest levels of f). The coefficients (β) were obtained from the regression model (Table 6) in order to verify the validity of the experiments. Besides, the experimental data were compared with the calculated values, Figure 2 shows that the parameters of the predicted and experimental values form a straight line, also is evaluated the confidence interval of 95% for the slope and intercept, suggesting that the statistical model (Eq. 7, Table 5) authenticates the factors that significantly contribute to the efficiency of the degradation.


Kinetics of phenol degradation by Fenton and Fenton modified reagents
The kinetic studies of phenol oxidation were carried out with the Fenton reagent (Fe2+/H2O2) and the Fenton modified reagents [Fe(terpy)Cl2]/H2O2 (Fig. 3) and [Fe(TBMA)Cl3∙3H2O]/H2O2. The oxidation behavior was analyzed by determining the substrate concentration at different time intervals (see experimental section). After analyzing the results, it was found that the substrate concentration decreases during the oxidation, indicating that the OH∙ radical produced from the Fenton or Fenton modified reagents effectively degrades phenol (Fig. 3).

Since the concentration of OH∙ radicals produced by the reaction of H2O2 with Fe(II) is difficult to determine, the oxidation kinetics of phenol was determined by equation 11 on the basis of substrate concentration.

Cphenol = phenol concentration, n = reaction order, k = reaction rate constant, and t = time. For the first-order reaction, equation 11 is modified to give equation 12.

Where Ci = initial concentration of phenol; Xphenol = reacted phenol fraction. The kinetic degradation of phenol (Figure 4a) by the Fenton reagent can be represented by plotting the concentration against different time intervals, i.e., f(C) = kt, (in a straight line plot of f(C) vs. t (time), a slope corresponds to the observed k), [28] confirming that the degradation of phenol follows a first-order reaction kinetics. However, for the treatment of phenol by Fenton modified reagent (Fe-complexes/H2O2) it follows second-order kinetics, as 1/C -1/Ci vs. time yields a straight line (Table 6). The second-order kinetic equation is given bellow:


For the three degradation processes it was found that the phenol concentration decreases drastically in the first 20 min of the reaction (up to 80% of degradation, Figure 4). Besides, an induction period was found in the conventional Fenton process (5 min), which means that as an initial stage, the reaction declines rapidly because of the Fe2+ consumption (Eq. 1). The reaction recovers through the reduction of Fe3+ to Fe2+ by hydroquinone. If the Fe2+ concentration is constant in all the process, the induction period is a stage that limits the reduction rate, and Fe3+ is reduced by hydroquinone to sustain the reaction. The rate constants of the Fe3+ reduction through benzoquinones (H2Q) and hydrogen peroxide have been determined by Chen et al., [29] and the evolution of the induction period to a progressive conversion period has been previously studied [24, 30].
H2Q + 2 Fe3+ + 2OH- → Q + 2Fe2+ + 2H2O (k = 4.4 × 10-2 L mol-1s-1) (9)
Fe3+ + H2O2 → Fe2+ + HO2 + (10)H+ (k = 1.0 × 10-2 L mol-1s-1) (10)
Also, is possible to observe with table 6 and equation 3, that decomposition by means of modified Fenton reagent, is proportional to [Cphenol]2, (second-pseudo order kinetic reaction with respect to phenol), while decomposition with conventional Fenton reagent is directly proportional to concentration of phenol, [Cphenol] (first-pseudo order kinetic reaction with respect to phenol), indicating that reactions with iron complexes is more efficient that conventional Fenton reaction, since with a lower concentration of Fe, is obtained a greater rate of degradation of phenol; thus a higher concentration of phenol will increase the decomposition rate with the same amount of Fe. Likewise, comparing the rate constants obtained from the reactions with iron complexes, is possible to note that reaction with [Fe(TBMA)Cl3∙3H2O] is faster than reaction with [Fe(terpy)Cl2] at the same reaction conditions.
Analysis of phenol degradation by conventional and modified Fenton process
The phenol oxidation, as observed in the experimental designing studies (Table 3), depends strongly on the experimental conditions; for example, for the conventional Fenton reaction, the concentration of Fe(II) shows the strongest influence on the degradation process, followed by pH, both consistent with previously reported results [31]. The phenol degradation was higher (98.0%) when the variables were: [Fe2+] = 8.9 × 10-5 mol L-1, [H2O2] = 8.8 × 10-4 mol L-1, pH = 4.6; however, it decreased to 21.69% when the concentrations of [Fe2+] and [H2O2] were 1.8 × 10-5 mol L-1, and 8.9 × 10-5 mol L-1, respectively, at pH = 3.0. This indicates that the increase of H2O2 in the reaction causes a negative effect in the degradation due to the fact that at high concentrations of hydrogen peroxide, the production of hydroxyl radicals becomes low because of the regeneration reaction (Eq. 14) that consumes H2O2 despite the formation of other radicals such as hydroperoxide radicals (HO2 ∙, Eqs. 15-16) from H2O2..However, if the concentration of Fe2+ is increased, the conversion of H2O2 into hydroxyl radicals is increased as well.
Fe3+ + H2O2 → Fe2+ + HO2∙ + H+ (14)
2H2O2 + 2HO∙ → 3HO2∙ + 3H+ (15)
H2O2 + HO∙ → HO2∙ + H2O (16)
In the same way as the Fenton reaction, for [Fe(terpy)Cl2]/H2O2, the high degradation rate of phenol was achieved when [Fe] and [H2O2] were at their highest level and pH at its lowest. In addition, the concentrations of H2O2 affect the oxidation process to a greater extent than pH, thus increasing phenol oxidation at low pH values. Likewise, with [Fe(TBMA)Cl3∙3H2O], pH strongly influences the oxidation process. In the processes with iron complexes no induction period was observed. However, although the kinetics of a unimolecular reaction can be fairly determined with one only exponential phase, if mediators are not detected, it does not mean that they do not exist. On the other hand, it means that, in case of existing, they disappear considerably more rapidly than they form.
Data analysis showed that pH is a vital factor that has a strong influence on the treatment of phenol by the Fenton process, which is consistent with previous results [23, 25, 26, 32-34]. Therefore, the ligand-based Fe complexes, instead of free Fe(II), become interesting to avoid the Fe(OH)3 precipitation and to increase the pH intervals, i.e., by chelating ligands with Fe(III) ion, OH-can be dispersed from entering towards Fe(III) ions, in such a way that Fe(OH)3 formation can be avoided. In previous studies, it has been shown that iron complexes, such as FeEDTA or iron oxyhydroxide ores [35], generate hydroxyl radicals under neutral pH conditions by reacting with H2O2 and they have been employed to oxidize the organic compounds. However, a value of pH 7.0 was used in the degradation of phenol to see its effect on the oxidation reaction (Figure 5). The conditions shown in Figure 5 were obtained through preliminary experiments.
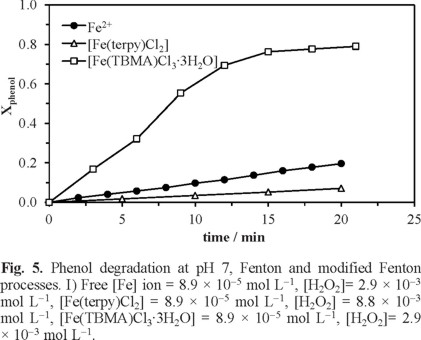
For example, with [Fe(terpy)Cl2],/H2O2, even at elevated concentrations of hydrogen peroxide (8.8 × 10-3 mol L-1, 300 mg L-1), the phenol degradation is very low. This indicates that the reaction is inefficient at high pH even if a high oxidation of phenol by Fe complexes using terpyridine was expected. This is probably due to the fact that the terpyridine ring, which has high stabilizing character, stabilizes strongly Fe(II) without permitting the oxidation to Fe(III) [36, 37]. A reaction is of order zero when the reaction rate is independent from the substance concentration, which indicates that the conversion is proportional to time. In the case of the process with Fe2+ and [Fe(terpy)]2+, the reaction rate is determined by pH, and not by the [Fe] and [H2O2] concentrations under the conditions studied here (see Figure 5). However, since benzimidazole moiety is a moderate σ- donor and ϖ- acceptor to the metal ion, the complex [Fe(TBMA)Cl3∙3H2O] /H2O2 effectively works at pH = 7.0 for the degradation of phenol (80%), although it requires an elevated dose of hydrogen peroxide (8.8 × 10-3 mol L-1, 300 mg L-1).
Conclusions
Experimental results showed that both complexes used had an excellent response in the phenol degradation. The best conditions of decomposition for modified Fenton reagent were when [Fe] and [H2O2] were in their maximum levels and pH in minimum level, i.e. the optimum reaction pH was 3.5 for both complexes. Unlike conventional Fenton reagent, the reactions with complexes had good response when the variables were in minimum levels, for both cases it was obtained a phenol decomposition of 70%, when for conventional Fenton reagent was only 20%. Besides, factorial analysis results showed that the reaction in the system [Fe(terpy)Cl2]/H2O2 is more dependent to the [H2O2], while the reaction in the system [Fe(TBMA)Cl3∙3H2O]/H2O2 was the pH in negative way (i.e. best response at low pH). Regarding the system Fe2+/[H2O2], it was found that the reaction was more dependent to the [Fe], coinciding with previous studies.
The results of the kinetics analysis showed that the reaction with iron complexes, to be more dependents to the phenol concentration, is possible to obtain a greater decomposition rate at higher concentrations of phenol. Of the three catalyst used, only Fe2+ presented an induction period; this stage is related with the high consumption of Fe2+ at the beginning of the reaction, then if the Fe concentration is constant along the reaction, the induction period is an stage that limits the reaction. Although both complexes can be used instead of Fe2+ at similar conditions of pH (3.5-4.5), only [Fe(TBMA)Cl3∙3H2O] showed excellent response at pH 7.0, even though was necessary a higher concentration of hydrogen peroxide. Some advantages found in this work for the modified Fenton reagent were: reactions with iron complexes are effective over a wider range of [Fe], [H2O2] and pH; the reaction is more dependent of the [phenol]; does not presents an induction period that limits the reaction rate; and [Fe(TBMA)Cl3∙3H2O] can be used at neutral pH, avoiding precipitation of Fe(OH)3.
Experimental Procedure
Reagents and Solvents
Phenol, hydrogen peroxide (30%), iron(II) chloride tetrahydrate, iron(III) chloride hexahydrate, ammonium hydroxide, monobasic potassium phosphate, dibasic potassium phosphate, 4-aminoantipyrine, potassium ferrocyanide, sodium hydroxide, nitrilotriacetic acid, 1,2-diaminobenzene, 2,2';6',2"-terpyridine (terpy) and methanol (ACS grade) were used as purchased from Sigma-Aldrich.
Physical Measurements
Elemental analyses for all the compounds were carried out On a Fisons Model EA 1108 CHNSO. 1H and 13C spectra were recorded for the compounds on a Varian Gemini (300 MHz) by using TMS as an internal standard. For the compounds, the electronic spectrum was measured with a Perkin–Elmer Lambda-900 double beam UV/Vis/NIR spectrophotometer; the mass spectral fragments were obtained in a Leco Pegasus III GC-TOFMS Mass Spectrometer. The pH was measured with an Orion 3 star pH-meter equipped with a Ross Ultra Sure Flow electrode. The matrix design of the factorial effects (23) was employed to estimate the effects of the variables. The normal probability plot was drawn by using all the calculated effects in MINITAB 15, Statistical Software.
Synthesis of tris(benzimidazol-2-ylmethyl)amine (TBMA)
The ligand TBMA was synthesized as described in the literature [31, 38-39]. A mixture of nitrilotriacetic acid (0.02 mol, 3.82 g) and 1,2-diaminobenzene (0.06 mol, 6.49 g) was refluxed for 24 h in hydrochloric acid (50 mL, 6.0 mol L-1) . The resulting solution was allowed to cool and the green crystals obtained were collected and washed with hydrochloric acid (2.0 mol L-1). The free base was isolated when the hydrochloride of the compound in water was neutralized with ammonium hydroxide (20%), then it was re-crystallized from aqueous ethanol and dried over P4O10. Yield 80%. Mass spectral fragments: (MS, ID, m/z (%)): MS, m/z 407 (M+)[C24H21N7]+, 132 (100) [C8H8N2]+, 146 [C8H8N3]+, 276 [C16H14N5]+. 1H NMR (300 MHz, CD3OD): δ 4.11 (s, 6H, methylene-N-), 6.77-6.46 (m, 15H, aromatic ring of benzimidazole). 13C NMR (300 MHz, CD3OD): δ51.77 (t, bzim-CH2-N), 126.56-155.85 (t, aromatic ring of benzimidazole). Elemental analysis calculated for C24H21N7∙2H2O: C, 64.99%; H, 5.68% and N, 22.11%. Observed: C, 64.84%; H, 5.51% and N, 21.74%.
Synthesis of iron complexes
[Fe(terpy)Cl2]: The compound was prepared as previously explained [40]. A solution of FeCl3 ∙4H2O (1.9 mmol, 0.378 g) dissolved in methanol was added to a solution of 2,2';6',2"-terpyridine (1.5 mmol, 0.35 g) dissolved in ethanol (30.0 mL) and the resulting solution was sonicated for 20 min, and then concentrated by removing excess methanol via rotary evaporation. The obtained residue was washed with diethyl ether and then re-crystallized from methanol (20 mL). A purple-colored solid was obtained (Yield: 78.3%). 1H NMR (DMSO-d6): δ9.19 (s, 2H, 3' & 5'), 8.86 (d, 2H, J = 7.8 Hz, 3 & 3''), 7.96 (t, 2H, J = 7.2 Hz, 4 & 4''), 7.19 (m, 4H, 5, 5'', 6 & 6''), 3.04 (s, 3H, Me). 13C NMR (DMSO-d6): δ119.0-121.0 (C4, C4'', C3', C5'); 121.0-122.5 (C6, C6''), 135.9-138.5 (C5,C5'',C4'); 149.0-150.0 (C3,C3'); 157.0-157.9 (C1,C1'', C6', C2'). Elemental analysis found for the compound coincides with the calculated values. C15H11N3FeCl2: Anal. Calc: C, 50.06%; H, 3.07% and N, 11.67%. Observed: C, 50.14 %; H, 3.13% and N, 11.59%.
[Fe(TBMA)Cl3∙3H2O]: The same procedure which was adopted for [Fe(terpy)Cl2] was used to prepare [Fe(TBMA)Cl3∙H2O] using N,N,N-tris(benzimidazol-2-ylmethyl)amine (0.025 mmol, 0.1075 g) with FeCl3 ∙4H2O (0.025 mmol, 0.0676 g). Yield: (87%). 1H NMR (CD3OD): δ(ppm) = 3.46-3.67 (s, 6H, -CH2-), 7.24-7.70 (m, 15H, benzimidazole-ring). 13C NMR (300 MHz, CD3OD): δ 49.14 (CH2-N-), 142.5-116.5 (benzimidazole-ring). Elemental analyses found for the compound coincide with the calculated values: C24H21N7FeCl3∙3H2O: Anal. Calc.: C, 46.21%; H, 4.33% and N, 15.73%. Observed: C, 45.97%; H, 4.35% and N, 15.43%. The presence of water molecules in the ligand and complex was determined by IR experiments. Also, physical and chemical properties of both iron complexes have been widely studied in previous works [31, 36-40].
Experimental design
The statistical design, which is an experimental planning process that provides information about the variables having a considerable effect on any systems, as well as the interaction between them, offers a simple regression model that combines the different independent variables and their interactions to predict the desired values in the response with a minimum amount of experiments. Furthermore, in the designing experiment and its statistical data analysis, the parameters such as factors and their levels, response variable, type of experimental design and data analysis are considerably important [41]. In this work, a factorial design at two levels [n = 2K, K = 3 (number of variables), n = 8, number of experiments)] was employed by considering three independent variables: (i) Concentration of iron [Fe] (A), (ii) reaction medium pH (B), and (iii) concentration of hydrogen peroxide [H2O2] (C). Therefore, the dependent variable is considered to be a selected response for the degradation of phenol (![]() ). The combinations of treatments (n = 8) can be represented as a cube model (Fig. 6).
). The combinations of treatments (n = 8) can be represented as a cube model (Fig. 6).

Degradation of phenol by the Fenton process
An aqueous phenol solution (1.0 × 10-4 mol L-1) was added to the Fenton solution (mixture of Fe (II) and the H2O2). A suitable pH (3.0 to 4.0) was adjusted by adding NaOH or H2SO4 (0.1 mol L-1). After mixing the phenol solution with the Fenton reagent (see Table 1), the oxidation of phenol was observed by colorimetric method, measuring the intensity of the phenol signal at 500 nm on a Perkin Elmer Lambda-900 spectrophotometer. A series of 8 experiments was carried out, and each was repeated with duplicate tests for the consistency of the data. It was found that there is an efficient oxidation of phenol with a reaction time of 15 min for conventional Fenton and 30 min for modified Fenton; these times for the processes were determined through previous experiments. In the colorimetric experiment, 4-aminoantipyrine was added to the substrate solution in order to see the phenol peak in the visible region [42]. After adjusting the pH of the sample solution (10 mL) by NH4OH to 7.9 ±0.1, 4-aminoantipyridine (0.1 mol L-1, 0.1 mL) was added, followed by addition of K3[Fe(CN)6] (0.24 mol L-1, 0.1 mL). The resulting solution, which was stirred for 15 min, turned to red and it was recorded on a UV/Vis spectrophotometer at 500 nm. The degradation of phenol was stopped with aqueous NaOH for the experiments.
Degradation of phenol by Fenton modified process
A Phenol solution, which was prepared by dissolving phenol into deionized water, was treated by the chelate TBMA or terpy based Fenton reagents. The reaction was carried out at different pH; in the same way, the pH of the solution was adjusted with H2SO4 or NaOH (0.1 mol L-1). The mixing time of the solutions (phenol and iron based compounds) was 5.0 minutes and then H2O2 was added to initiate the oxidation of phenol. The concentration of phenol was measured by the colorimetric method as described above for the Fenton reagent. Rate constants for the oxidation of phenol were determined by plotting the substrate concentrations against different time intervals.
Acknowledgments
The authors would like to thank the Facultad de Química of the Universidad Nacional Autónoma de México for allowing us to work in the inorganic and nuclear chemistry laboratory (F-114). We would also like to thank the Unidad de Servicios de Apoyo a la Investigación USAI, UNAM.
References
1. Salkinoja, S. M.; Uotila, J.; Jokela, J.; Laine, M.; Saski, E. Environ. Health Persp. 1995, 5, 63. [ Links ]
2. Zhao, F.; Mayura, K.; Hutchinson, R. W.; Lewis, R. P.; Burghardt, R. C.; Phillips, T. D. Toxicol. Lett. 1995, 78, 35. [ Links ]
3. 5. Luis, A.; Lombraña, J. I.; Varona, F.; Menéndez, A. Korean J. Chem. Eng. 2009, 26, 48. [ Links ]
4. Busca, G., Berardinelli, S., Resini, C., Arrighi, L. J. Hazard. Mater. 2008, 160, 265-288. [ Links ]
5. Staehelin, J.; Hoigne, J. Environ. Sci. Technol. 1985, 19, 1206. [ Links ]
6. Ollis, D. F.; Pelizzetti, E.; Serpone, N. Heterogeneous photocatalysis in the Environment: application to water purification. In Photocatalysis: Fundamentals and Applications, ed. N. Serpone and E. Pelizzetti. Wiley Interscience, New York 1989, 603. [ Links ]
7. D' Oliveira, J. C.; Al-Sayyed, G.; Pichat, P. Environ. Sci. Technol. 1990, 24, 990. [ Links ]
8. Okamoto, K.; Yamamoto, Y.; Tanaka, H.; Itaya, A. Bull. Chem. Soc. Jpn. 1985, 58, 2023. [ Links ]
9. Wong, A. S.; Crosby, D. G. Photolysis of pentachlorophenol in water, in: K.R. Rao (Ed.), Pentachlorophenol, Chemistry, Pharmacology, and Environmental toxicology, Plenum Press, New York, 1978, 19. [ Links ]
10. Choudhry, G. G.; Hutzinger, O. Residue Reviews 1982, 84, 133. [ Links ]
11. S. Vollmuth, A.; Niessner, Z. R. Environ. Sci. Technol. 1994, 28, 1145. [ Links ]
12. Chen, D.; Ray, A. K. Water Res. 1998, 32, 3223. [ Links ]
13. Davis, A. P.; Huang C. P. Water Res. 1990, 24, 543. [ Links ]
14. Lu, M. C.; Roam, G. D.; Chen, J. N.; Huang, C .P. J. Photochem. Photobiol. A: Chem. 1993, 76, 103. [ Links ]
15. Pelizzetti, E.; Pramauro, E.; Minero, C.; Sepone, N.; Borgarello, E. Photodegradation of organic pollutants in aquatic systems catalyzed by semiconductors, in: M. Schiavello (Ed.), Photocatalysis and Environment, Trends and Application, NATO ASI Series C, Kluwer Academic Publishers, Dordrecht, The Netherlands 1998, 237, 469. [ Links ]
16. Haag, E. R.; Yao, C. C. D. Environ. Sci. Technol. 1992, 26, 1005. [ Links ]
17. Mills, G.; Hoffmann, M. R. Environ. Sci. Technol.1993, 27, 1681. [ Links ]
18. Martin, S. T.; Morrison, C. L.; Hoffman, M. R. J. Phys. Chem. 1994, 98, 13695. [ Links ]
19. Barbeni, M.; Minero, C.; Pellzzetti, E. Chemosphere 1987, 16, 2225. [ Links ]
20. Nam, K.; Rodriguez W.; Kukor, J. J. Chemosphere 2001, 5, 11. [ Links ]
21. Martins, R. C.; Rossi, A. F.; Quinta-Ferreira, R. M. J. Hazard. Mater. 2010, 180, 716. [ Links ]
22. Bianco, B.; Michelis, I.; Vegliò, F. J. Hazard. Mater. 2011, 186, 1733. [ Links ]
23. Lin S. H.; Lo C. C. Water Res. 1997, 31, 2051. [ Links ]
24. Morales-Roque, J.; Carrillo-Cárdenas, M.; Jayanthi, N.; Cruz J.; Pandiyan, T. J. Mol. Struct. (Teochem) 2009, 910, 74. [ Links ]
25. Kwon, B. G.; Lee, D. S.; Kang, N.; Yoon, J. Water Res. 1999, 33, 2110. [ Links ]
26. Busca, G.; Berardinelli, S.; Resini C.; Arrighi, L. J. Hazard. Mater. 2008, 160, 265. [ Links ]
27. Pignatello, J. J. Environ. Sci. Technol. 1992, 26, 944. [ Links ]
28. Levenspiel, O. Ingeniería de las Reacciones Químicas, 3th ed.; Reverte: España 2010, chapter 2. [ Links ]
29. Chen, R.; Pignatello, J. J. Environ, Sci. Technol. 1997, 31, 2399. [ Links ]
30. Northup A.; Cassidy, D. J. Hazard. Mater. 2008, 152, 1164. [ Links ]
31. Takahashi, K.; Ogawa, E.; Oishi, N.; Nishida Y.; Kida, S. Inorg. Chim. Acta 1982, 66, 97. [ Links ]
32. Teel, A. L.; Watts, R. J. J. Hazard. Mater. 2002, B94, 179. [ Links ]
33. Badawy, M. I.; Ghaly M. Y.; Gad-Allah, T. A. Desalination 2005, 194, 166. [ Links ]
34. Khan, E.; Wirojanagud W.; Sermsai, N. J. Hazard. Mater. 2009, 161, 1024. [ Links ]
35. Goldstein, S.; Meyerstein, D.; Czapski, G. Free Radical Bio. Med. 1993, 15, 435. [ Links ]
36. Judge, J. S.; Reiff, W. M.; Intille, G. M.; Ballway, P.; Baker, W. A. J. Inorg. Nucl. Chem. 1967, 29, 1711. [ Links ]
37. Brandy, W. W.; Wright, J. P. J. Am. Chem. Soc. 1954, 76, 3082. [ Links ]
38. Morales-Roque, J.; Pandiyan, T.; Cruz J.; García-Ochoa, E. Corros. Sci. 2008, 50, 614. [ Links ]
39. Thompson, L. K.; Ramaswamy B. S.; Seymour, E. A. Can. J. Chem. 1977, 55, 878. [ Links ]
40. Reiff, W. M.; Erickson N. E.; Baker Jr., W. A. Inorg. Chem. 1969, 8, 2019. [ Links ]
41. Montgomery, D. C. Diseño y análisis de experimentos, 2nd ed.; Limusa Wiley: México, 2005, chapter 9. [ Links ]
42. Franson, M. A. H. Métodos Normalizados para el análisis de aguas potables y residuales, APHA-AWWA-WPCF, 17th ed.; Díaz de Santos: España 1992, chapter 5. [ Links ]














