Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.57 no.2 Ciudad de México abr./jun. 2013
Article
Adsorption of Chromium(VI) on Radiation Grafted N,N-dimethylaminoethylmethacrylate onto Polypropylene, from Aqueous Solutions
Guillermina Burillo,1 Juan Serrano-Gómez,*2 and Juan Bonifacio-Martínez2
1 Instituto de Ciencias Nucleares, Universidad Nacional Autónoma de México. Departamento de Química, Ciudad Universitaria, México 04510 D.F. México.
2 Instituto Nacional de Investigaciones Nucleares. A. P. 18-1027. Col. Escandón. México, D. F. 11801, México. juan.serrano@inin.gob.mx
Received April 19, 2013
Accepted April 5, 2013
Abstract
Polypropylene (PP) grafted with dimethylaminoethylmethacrylate (DMAEMA), was prepared by irradiation with a 60Co γsource. The obtained PP-g-DMAEMA was used to study the Cr(VI) ion adsorption as a function of contact time, initial pH, initial concentration of metal ion and temperature. Chromium adsorption data on PP-g-DMAEMA at various initial concentration fit well the Freundlich and Langmuir isotherms. The maximum adsorption capacity (amax) was found to be 0.3103 × 0-4 mol g-1. The thermodynamic parameters ΔH0, ΔG0 and ΔS0 were estimated showing the adsorption process to be exothermic and spontaneous.
Key words: Radiation grafting, DMAEMA, adsorption, thermodynamics, chromium.
Resumen
Se preparó polipropileno (PP) injertado con dimetilaminoetilmetacrilato (DMAEMA) por irradiación con una fuente de radiación gamma de 60Co. El PP-g-DMAEMA se usó para estudiar la adsorción de iones de Cr(VI) como una función del tiempo de contacto, pH inicial, concentración inicial del ión metálico y la temperatura. Los datos de adsorción de cromo en PP-g-DMAEMA a varias concentraciones iniciales se ajustaron bien a las isotermas de Freundlich y Langmuir. Se encontró que la capacidad de adsorción máxima (amax) fue de 0.3103 × 10-4 mol g -1. Se calcularon los parámetros termodinámicos ΔH0, ΔG0 y ΔS0, los cuales indican que el proceso de adsorción es un proceso exotérmico y espontáneo.
Palabras clave: Injerto por radiación, DMAEMA, adsorción, termodinámica, cromo.
Introduction
Removal and recovery of heavy metals from industrial discharges is an economic and environmental problem. Toxic metals, such as chromium, should be removed before coming in contact with the environment. Chromium is a metal that exists in various oxidation states, and in aqueous solutions the most stable of them are the hexavalent, Cr(VI), and the trivalent, Cr(III) states. Hexavalent chromium compounds are significantly more toxic than trivalent ones, even at low concentrations, and they have a potential carcinogenic effect. Before discharging effluents contaminated with chromium to the environment, the content of this toxic element should be reduced to the allowable limit of 0.1 mg L-1. Inorganic [1-5], organic and bio-materials [6-11] have been investigated to remove toxic metals from aqueous systems. Among the organic materials, polymers obtained by radiation processing of grafting and crosslinking [12-16] have shown high performance for that purpose. However, most of the papers in the literature about the use of grafted and crosslinked polymers to remove heavy metals are devoted to metals that form cations in solution, and a very small number of papers report about toxic anion separation.
Radiation graft polymerization has many advantages over other conventional methods, such as, chemical and photochemical grafting. For instance, the method is relatively simple and no catalyst or additives are needed to initiate the reaction. It has been used for modification of polymeric materials; including polypropylene (PP), polyethylene (PE), poly ethylene terephthalate (PET) [17-18]. Dimethylaminoethylmethacrylate (DMAEMA) is a pH responsive monomer, its amine groups causes stronger hydrophobic interactions at high pH, and undergoes an abrupt precipitation above pH 5.4 named critical pH point [19]. In DMAEMA grafted into PP (PP-g-DMAEMA) films, the critical pH point shifted to a lower pH of 5. This system shows reversibility of swelling-deswelling behavior by varying the pH from 2.2 to 9 or the temperature from 293 to 323 K. DMAEMA is also a temperature stimuli-responsive monomer, it exhibits a low critical solution temperature (LCST) in the range of 311-313 K. The polymer is soluble below the LCST, while above this transition temperature, they become increasingly hydrophobic and insoluble. The aim of this work was to synthesize polypropylene (PP) films grafted with DMAEMA, by gamma radiation and to study the removal of Cr(VI) anions from aqueous solutions through their sorption on the grafted system and the parameters that affect the chromate adsorption process, such as contact time, pH, chromium concentration, and temperature.
Results and Discussion
Grafting
The grafting yields of DMAEMA onto PP increases with increasing radiation absorption up to 30 kGy, and then levels off [18]. It is known that PP is a radiation crosslinkable polymer but essential changes in its structure, caused by γradiation, occur at doses of about 100 kGy and more [20]. Initial results showed that maximum uptake of Cr(VI) ions was obtained at radiation grafting percentages of DMAEMA about 100%. To have these percentages the samples were radiation grafted at dose rate of 8.6 kGy h-1 with an absorption dose of 11 kGy and a monomer concentration of 50%vol. To carry out the batch sorption experiments, the PPg-DMAEMA foils were cut into pieces (about 3x3 mm) and then thoroughly mixed. Taking into account the grafting percentage of all the cut foils the average grafting percentage of DMAEMA samples used in the sorption experiments was 103.74 ± 5.06. FTIR spectra confirm the grafting reaction of DMAEMA onto PP. Characteristics absorption bands of PP were –CH3 (2917 and 1456 cm-1); but after the DMAEMA grafting an additional peak of –C=O (1625 cm-1) appeared (Fig. 1).

Effect of contact time
The initial pH of the 1 × 10-4 M K2CrO4 solution utilized in the batch experiments was 5.5. Figure 2 shows a plot of q versus shaking time, where q is the amount of chromate ions (in mmol) adsorbed per gram of PP-g-DMAEMA, at any time. The results shown in Figure 2 indicate that the Cr(VI) adsorption on the polymeric system PP-g-DMAEMA was rather slow and reached complete equilibrium within 10 hours of shaking time. At equilibrium, the adsorption of Cr(VI) on the adsorbent was found to be 6.44 × 10-3 mmol g-1.

Effect of initial pH
The influence of initial pH on the chromium ions adsorption onto the PP-g-DMAEMA was studied by using a 1 × 10-4 mol L-1 KxCrO4 aqueous solution, and the batch experiments were carried out at 293 K. No buffer was added to chromium solution in order to avoid the presence of any external electrolyte, which may influence the adsorption process. The initial pH of the Cr(VI) solutions was adjusted in the range from 2 to 12 by using concentrated HCl and a concentrated NaOH aqueous solution. The obtained results are shown in Figure 3 where ae, the amount of chromate ions adsorbed at equilibrium per gram of PP-g-DMAEMA, has been plotted versus the initial pH. The chromate ion retention as a function of initial pH showed a maximum value at an initial pH 2.0 and then decreased as the initial pH was increased. PDMAEMA is a polymer that is both thermosensitive with a low critical solution temperature (LCST) around 311 K, and pH sensitive with a pH critical point of 5.4 [19]. It was observed by Burillo [21] that DMAEMA grafted onto PP which exhibit a LCST transitions at neutral pH, also exhibit hydration transitions at low pH that correspond to UCST-type behavior; that is, a hydrophilic phase at pH 2 and hydrophobic phase at pH 6 or more. At pH 2.0 and with a chromium concentration of 1.0 × 10-4 mol L-1 , Cr(VI) ions exist mainly (about 99.9 %) as the anion HCrO4 with a negligible amount of H2CrO4, as it was showed in the equilibrium diagram of chemical species of Cr(VI) as a function of pH (Fig. 4). The equilibrium diagram was obtained by the Program Medusa [22] for the Cr(VI) concentration just mentioned. At pH 2, groups of the DMAEMA like _N(CH3)2 present in the grafted polymer acquire a positive charge because of the attachment of a proton. Then, the chromium adsorption may occur through reactions 1 and 2, forming an ionic bond between the positive charged amino groups and HCrO4- ions:
(CH3)2_N_R + H+ ↔ (CH3)2_NH+_R (1)
(CH3)2_NH+_R + HCrO4- ↔ ((CH3)2_NH+_R)(HCrO4-) (2)


where R represents the carbon chain of the grafted polymer. At pH 4, Cr(VI) is found only as HCrO4- ions in the aqueous solution [22]. At pH 6, besides HCrO4- ions, CrO42- ions start appearing in the aqueous solution. Reaction (3) and (4) describe the adsorption reaction of CrO42- ions onto the grafted polymer.
(CH3)2_N_R + H+ ↔ (CH3)2_NH+_R (3)
2(CH3)2_NH+_R + CrO42- ↔ ((CH3)2_NH+_R)2(CrO42-) (4)
At pH 8, Cr(VI) is present mainly as CrO42-ions and from 9 to 12 only CrO42-ions [22] can be observed in the solutions. As pH is increased, the number of electrically charged positive active sites decreases strongly, which can explain the drastic reduction of chromium retention on the grafted polymer at high alkaline pH values.
Effect of chromium concentration
Adsorption Isotherms
The capacity of an adsorbent can be described by equilibrium sorption isotherm, which is characterized by certain constants whose values express the surface properties and affinity of the adsorbent. The sorption isotherms were investigated using two equilibrium models, which were namely the Freundlich and Langmuir isotherm models since they give valuable information about the sorption process. Thus, the effect of chromate ion concentration on its adsorption onto the grafted polymer was studied by carrying out batch experiments at 293 K and 24 h of shaking time. Solutions with the desired concentrations of chromium anions were prepared by successive dilutions of a stock solution of 1.0 × 10-3 mol L-1 K2CrO4 in distilled water. In Figure 5 the logarithm of ae has been plotted versus the logarithm of Ce. The obtained straight line is described by Freundlich equation:
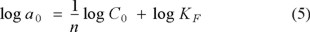

where Ce is the equilibrium concentration of solution (mol/L), KF and 1/n (0 < 1/n < 1) are the Freundlich constants corresponding to the adsorption capacity and intensity of adsorption, respectively, and ae was already defined above. These constants can be estimated by the intercept and the slope (less than 1) of the straight line, respectively. The values of KF and 1/n, computed by using the least square technique, were found to be 1.05 × 10-4 mol g-1 and 0.20, respectively, with a correlation coefficient of 0.9963. The value of 1/n (less than one) found in this work confirms that the Freundlich isotherm is valid for the Cr(VI) adsorption data on the grafted polymer. The value 1/n, less than unity, is attributed to a heterogeneous surface structure of the adsorbent and also indicates an exponential distribution of energy sites [23]. The linear form of the Freundlich isotherm indicates a physisorption process [24] in the Cr(VI) sorption reaction with PP-g-DMAEMA.
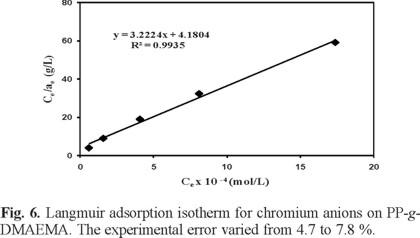
Langmuir isotherm was also used to test the Cr(VI) adsorption data on the grafted polymer. Langmuir isotherm equation is based on monolayer sorption onto a surface with finite number of identical sites. These identical sites are homogeneously distributed over the sorbent surface. The Langmuir isotherm in its linearized form is as follows:

where k and amax are Langmuir constants related to the adsorption energy and the maximum adsorption capacity, respectively. Figure 6 shows the linear plot obtained when Ce/ae was plotted versus Ce. The slope of this plot gives a maximum adsorption capacity (amax) equal to 0.3103 × 10-4 mol g-1, while the intercept yields the value of k = 3.223 × 104 L mol-1, and R2 = 0.9946. For the Cr(VI) sorption on PP-g-DMAEMA the Freundlich isotherm has a higher correlation coefficient than the Langmuir isotherm, which indicates that the Cr(VI) sorption data fitted the Freundlich isotherm better than the langmuir isotherm.
Effect of Temperature
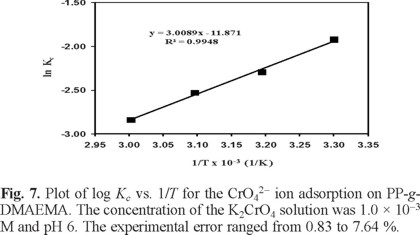
To investigate the temperature effect on chromium adsorption, batch experiments were carried out at 303, 313, 323 and 333 K with an initial Cr(VI) ion concentration of 1 × 10-4 M at pH 5.5 (near of the critical pH of 5), a contact time of 24 h and an adsorbent amount of 0.1 g. It was found that the amount of Cr(VI) adsorbed at equilibrium at various temperatures decreases with increasing temperature from 303 to 333 K. That is because the films were thermosensitive, and with an LCST of about 302 K, the swelling percentages decrease abruptly. Above this temperature the polymeric system was collapsed. The thermodynamic parameters (ΔG0, ΔH0 and ΔS0) for the adsorption of Cr(VI) on the polymeric system PP-g-DMAEMA were calculated using equilibrium constant, Kc [9]:

where ae and Ce have the same meaning as in equation (5) and (6). Thus, ΔH0 and ΔS0 were obtained from the slop and intercept of van't Hoff plots of ln Kc versus 1/T (Figure 7):

where T is the absolute temperature in degrees Kelvin and the gas constant R = 8.31434 kJ mol-1K-1.
The adsorption standard free energy changes ΔG0 can be calculated according to Eq. (9)
ΔG0= -RT ln Kc (9)
Table 1 shows the thermodynamic parameters of the chromate ions adsorption process. The values of ΔH0 and ΔS0 as obtained by using equation (8) were –57.61 kJ mol-1 and -227.31 J K-1 mol-1, The negative value of DH0 shows that the Cr(VI) sorption process is exothermic in nature. The negative value of DS0 suggests that the adsorbed solvent molecules displaced from the adsorbent by chromate ions gain more translational entropy than is lost by the chromate ions. The values of ΔG0 obtained at 30, 40, 50 and 60oC are all positive, which indicates that the adsorption process involved in the Cr(VI) sorption on PP-g-DMAEMA is not spontaneous. The increasing in the change of the standard free energy with the rise in temperature suggests that a better adsorption is actually obtained at low temperatures.

Conclusions
pH-sensitive and thermosensitive grafted films have been prepared by the irradiation-induced graft copolymerization of DMAEMA onto PP film (direct method). The chromium adsorption in the synthesized system was studied and found to decrease with temperature; further, it is at a minimum above the LCST of 302K. The optimum pH for the anion retention was found to be below the critical pH of the system, which presents an opposite behavior (high swelling at low pH and low swelling above the critical pH of 5). The study showed the ability of PP-g-DMAEMA to adsorb Cr(VI) ions from aqueous solutions. The adsorption in the equilibrium was found to be dependent on the initial concentration and temperature. Experimental adsorption isotherms of Cr(VI) ions on PP-g-DMAEMA fit well the Freundlich and Langmuir isotherms; while the negative value of the thermodynamic parameters DH0 indicate that the Cr(VI) ion adsorption process is exothermic, while the positive values of DG0 suggests that the adsorption process involved is not spontaneous.
Experimental
Materials
The monomer (DMAEMA) was purchased from Aldrich Co., USA and purified by distillation under reduced pressure. (0.03 mmHg). Isotactic polypropylene (PP) films of 1 × 5 cm, 60 µm-thick and 71% crystallinity from PEMEX México were washed in methanol for 24 h and then dried under reduced pressure (0.06 mm Hg) to a constant weight.
Radiation Grafting
PP films were placed in glass ampoules which contained DMAEMA (50% V/V solution in toluene). The reaction mixtures were de-aerated under vacuum by the method of freezing and thawing. The ampoules were then sealed and irradiated with a 60Co γ source (Gammabeam 651 PT, Nordion International Inc.) at a dose rate of 5.5 kGy h-1 and doses from 1 to 40 kGy. The residual monomer and homopolymer formed during the irradiation were extracted with refluxing toluene for 10 h [21]. The films were then vacuum dried at 40 oC and the grafted percentage was estimated applying the equation: G(%) = [(Wf -Wi)/Wi] × 100; where Wf and Wi are the weight of PP film after and before grafting, respectively.
Equilibrium water absorption time
Dried grafted films were immersed in distilled water at 293 K; the weights of samples were measured at various time intervals after soaking in water, and after the excess surface water was removed. The procedure was repeated until there was no further weight increase. The weight gain percentage was calculated by the equation: DWw (%) = [(Wf -Wi)/Wi] × 100 ; where Wf and Wi are the weights of the water soaked sample at time t and of the dried film, respectively. Once equilibrium was reached, the maximum weight gain (%Ww) was estimated. The equilibrium swelling time of the samples was reached within 3 h. The pH sensitivity was found by swelling measurements at pH values from 2 to 9, and the critical pH point was evaluated at the inflection point of the plot of swelling percentage as a function of pH, at room temperature. PP-g-DMAEMA samples have a critical pH point of 5.
Batch Cr(VI) adsorption experiments
Batch-type experiments were carried out at 293 K to determine the kinetic removal of Cr(VI) ions. Small flakes (100 mg) of PP-g-DMAEMA were shaken in closed vials with 10 mL aliquots of 1.0 × 10-4 mol L-1 Cr(VI) ions aqueous solution, which had a pH value of 5.5. Time intervals from 5 min to 24 h were used to attain the equilibrium distribution; then the liquid phase was recovered for chromium measurements. All of the experiments were performed in duplicate, by running two independent closed vials, simultaneously. The adsorption capacity of Cr(VI) ions by the grafted polymer was determined from the difference between the initial and final concentrations of Cr(VI) ions in the aqueous solutions, using a Shimadzu ultraviolet-visible 265 spectrophotometer analyzer at λ= 370 nm. For the chromium concentration studies, solutions with the desired concentrations of Cr(VI) ions were prepared by successive dilutions of a stock solution of 1.0 × 10-3 mol L-1 K2CrO4 in distilled water.
Acknowledgements
The authors wish to thank Francisco García from ICN, UNAM and Marcelino Villa Tomasa and Iris Z. López from the Chemical Department, ININ for technical assistance, and DGAPA UNAM grant IN200210 for economical support.
References
1. Jha, V. K.; Matsuda, M.; Miyake, M. J. Hazard. Mater. 2008, 160, 148-153. [ Links ]
2. Babel, S.; Kurniawan, T. A. J. Hazard. Mater. 2003, 97, 219-243. [ Links ]
3. Sayari, A.; Hamoudi, S.; Yang, Y. Chem. Mater. 2005, 17, 212-216. [ Links ]
4. Lalvani, S. B.; Wiltowski, T.; Hübner, A.; Weston, A.; Mandich, N. Carbon. 1998, 36, 1219-1226. [ Links ]
5. Lehmann, M.; Zouboulis, A. I.; Matis, K. A. Chemosphere 1999, 39, 881-892. [ Links ]
6. Sengupta, A. K.; Zhu, Y. AIChE. 1992, 38, 153-157. [ Links ]
7. Babu, B. V.; Gupta, S. Adsorption 2008, 14, 85-98. [ Links ]
8. Wang, J.; Chen, C. Biotechnol. Adv. 2009, 27, 195-226. [ Links ]
9. Ucun, H.; Bayhan, K. Y.; Kaya, Y. J. Hazard. Mater. 2008, 153, 52-59. [ Links ]
10. Tewari, N.; Vasudevan, P. Biochem. Eng. J. 2005, 23, 185-192. [ Links ]
11. Mahramanlioglu, M.; Bicer, I. O.; Misirli, T.; Kilislioglu, A. J. Radianal. Nucl. Chem. 2007, 273, 621-624. [ Links ]
12. Coşnuk, R.; Soykan, C.; Saçak, M. Sep. Purif. Technol. 2006, 49, 107-114. [ Links ]
13. Hegazy, E. A.; Abd El-Rehim, H. A.; Shawky, H. A. Rad. Phys. Chem. 2000, 57, 85-95. [ Links ]
14. Bhattacharya, A.; Misra, B. N. Prog. Polym. Sci. 2004, 29, 767-814. [ Links ]
15. Seko, N.; Tamada, M.; Yoghi, F. Nucl. Instrum. and Methods in Phys. Res., Sect. B: Beam Interact. with Mater. and Atoms. 2005, 236, 21-29. [ Links ]
16. Özeroglu, C.; Keceli, G. Radiochim. Acta 2009, 97, 709-717. [ Links ]
17. Chapiro, A. Radiation Chemistry of Polymeric System. Interscience, New York, 1962. [ Links ]
18. Bucio, E.; Aliev, R.; Burillo, G. Polymer. Bull. 2002, 45, 571-577. [ Links ]
19. Contreras-García, A.; Burillo, G.; Aliev, R.; Bucio, E. Radiat. Phys. Chem. 2008, 77, 936-940. [ Links ]
20. Woods, R. J.; Pikaev, A. K. Applied Radiaton Chemistry: Radiation Processing. John Wiley & Sons, New York., 1994. [ Links ]
21. Burillo, G.; Bucio, E.; Arenas, E.; López, G. Macromol. Mater. Eng. 2007, 292, 214-219. [ Links ]
22. Puigdomenech, I.: Program MEDUSA (make equilibrium diagrams using sophisticated algorithms), http://www.inorg.Kth.se/Reserach/Ignasi;/index.html. [ Links ]
23. Benes, P.; Majer, V. Trace Chemistry of Aqueous Solutions. General Chemistry and Radiochemistry. Elsevier Scientific Publishing Company, Amsterdam, 1980. [ Links ]
24. Ravichandran, P.; Hasmath Farzana, M.; Meenakshi, S. Indian J. Chem. Technol. 2012, 19, 103-110. [ Links ]














