Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.57 no.1 Ciudad de México ene./mar. 2013
Article
The Role of Bromination Reaction in the Oxalic Acid-Acetone-KBrO3-Ce(IV)-H2SO4 Oscillating System
Jinzhang Gao,* Yingying Zhang, Jie Ren, Wu Yang
College of Chemistry & Chemical Engineering, Northwest Normal University Lanzhou 730070, P. R. China. jzgao@nwnu.edu.cn
Received August 3, 2012.
Accepted March 7, 2013.
Abstract
In order to assess the role of bromination reaction in the oxalic acid-acetone-KBrO3-Ce(IV)-H2SO4 oscillating system, both the effects of oxalic acid and acetone were studied in detail. Based on the experimental data, we propose a new skeleton scheme containing 11 reactions to simulate the oscillating behavior of the system, in which the bromination reaction between acetone and bromine should be considered as another source of Br−.
Key words: Bromination Reaction; B-Z Oscillating Reaction; FKN Mechanism; Oregonator Model; Explodator Model; Double Organic Substrates.
Resumen
Se estudian en detalle los efectos del ácido oxálico y de la acetona en la reacción ácido oxálico-acetona-KBrO3-Ce(IV)-H2SO4 con el fin de evaluar el papel de la reacción de bromación el sistema oscilante. Basados en los datos experimentales, proponemos un nuevo esquema que contiene a 11 reacciones para simular el comportamiento oscilante del sistema en el que la reacción de bromación entre acetona y bromo debe ser considerada como otra fuente de Br−.
Palabras clave: Reacción de bromación, reaction oscilante B-Z, mecanismo FKN, modelo Oregonator, modelo Explodator, sustratos orgánicos dobles.
Introduction
The core of classical Belousov-Zhabotinskii (B-Z) reaction was considered as a redox system consisting of malonic acid (reductant) and potassium bromate (oxidant) in a strongly acidic medium (sulfuric acid) by using a catalyst (cerium ion Ce4+) to speed up the rate of reaction [1]. This system can display some oscillating behaviors due to the potential changes of Ce4+/Ce3+ and Br2/Br− alternately and has been widely studied as an analytical tool [2-4]. A large number of investigations indicated that any organic compounds containing active methylene could replace malonic acid as reductant and some metal ions or complexes such as Fe3+/Fe2+, Mn3+/Mn2+ etc., which have the electrode potential in the range of 1.5 V-1.0 V, could also be considered as catalyst in the B-Z oscillating chemical reactions. That is to say, the definition of classical B-Z reaction has been widened today. For illustrating these oscillators, many hypotheses and models were proposed, in which the FKN mechanism and Oregonator model were, perhaps, accepted generally [5-7].
Commonly, there are three cycles in FKN mechanism concerning 10 elementary reactions and 14 intermediate species [5], and the whole reactions are controlled by the interactions of three processes, in which the potential changes of Ce4+/Ce3+ and Br2/Br− alternate to display the oscillating behaviors. The FKN mechanism is fundamentally dependent upon the control of oscillation behaviors by the concentration change of bromide ion, which was consumed in Process A while regenerated in Process C. In the view of this mechanism, the typical oscillation system could be called the bromate-driven oscillator controlled by bromide. This idea has been recognized and used to explain successfully some non-linear behaviors observed [8-10].
Then, the prominent question for FKN mechanism was discovered in the non-bromide-ion controlled oscillations by Noszticzius et al. [11]. This discovery has led to the proposal of a B-Z mechanism being different from the FKN mechanism, in which it relies upon Br2 control rather than Br− control of the oscillations [12-13]. In addition, Noszticzius et al. [13] proposed another problem about the value of f, where f is a stoichiometric factor, meaning the number of bromide ions regenerated in Process C. The oscillations will fail to occur if f < 0.25 [14], however, a partial oxidation of bromomalonic acid alone requires 4 Ce4+ and produces only one bromide ion. Meanwhile, some organic compounds also react with Ce4+ but no bromide ions yield, such as malonic acid [14], will result in an f below the critical value of 0.25. That is, the redox reaction between Ce4+ and bromomalonic acids is not the only source of Br−. All of these are not fit well into the FKN framework to make researchers doubt the correctness of FKN mechanism, especially, on the key intermediate and the source of Br− ion. For this purpose, Noszticzius et al presented an essential modification of the FKN mechanism to obtain the Explodator model for the B-Z reaction [13], in which an alternative mechanism was used to avoid such pitfalls as step (O5) of the Oregonator. The dynamical structure of the Explodator relies on the interaction of two autocatalytic reactions E1 and E3-E2 to induce oscillations. Thus, it is profoundly different from the Oregonator which relies on the bistability implicit in Processes A and B and switching between them for the generation of oscillation. In 1985, Field [15] re-examined the mechanism of oxalic acid-acetone-KBrO3-Ce(IV)-H2SO4 oscillating system and proposed an Oregonator-like model which was based on 31 elementary reactions including 9 of reversible reactions. According to this model, the bromine-hydrolysis reaction is the main source of Br− ion and the oscillation system is still controlled by bromine or bromide ions. In acid solution, bromine-hydrolysis reaction is non-neglect factor, and the process of Br2 converting into Br− is easy. Noszticzius et al. [16] confirmed that only the Br− ion can react directly with the bromous and the elementary Br2 reacts indirectly via its hydrolysis.
According to the Oregonator-like model, oscillation behaviors could be simulated by means of mathematics. Results showed that the simulated behaviors were qualitatively consistent with experimental observations basically; however, the inconsistencies exist in the simulation shapes, such as the great gap of oscillation curve. In addition, the regeneration reaction of bromide ions is ambiguous. Although Explodator model has been successful used to explain the behaviors of the non-bromide-ion controlled oscillations, the feedback of metal ion catalyst was somewhat weakened [17]. Then, Gyorgyi, Turanyi and Field [8] re-proposed a new expanded FKN mechanism which contains 80 elementary reactions and 26 variable species concentrations. Indeed, the new model is much better than the original one. It not only incorporates the most recent suggestions as well as previous revisions of the original FKN mechanism but also confirms that B-Z oscillations in 1 M sulfuric acid medium still seem to be primarily bromide ion controlled and the role of organic radicals could be the secondary factor. Based on the expanded FKN mechanism, the source of bromide ion could be considered from the reaction between oxybromine compounds and organic radicals. Moreover, some reports implied that the bromination reaction between acetone and bromine was also an important source of Br−ions [18].
For understanding more easily this reaction, in the present paper, we offer a new skeleton scheme including 11 reactions, in which the bromination reaction between acetone and bromine was considered as another source of Br−, and investigated the oxalic acid-acetone-KBrO3-Ce(IV)-H2SO4 oscillating system again. The effect of bromination reaction in the regeneration of Br− was examined via calculating the baseline concentration of Br− in different steady states.
Experimental section
Reagents
All chemicals used to establish B-Z oscillating system such as KBrO3, acetone, oxalic acid, H2SO4, and Ce(SO4)2·H2O were of analytical grade. Doubly distilled water was used for solution preparation. Solutions of KBrO3, Ce(SO4)2, acetone, and oxalic acid were prepared in 1.0M sulfuric acid.
Apparatus
The experiment was performed in a closed thermostat-regulated glass container (ca. 50 mL) equipped with a magnetic stirrer for homogenization. A CHI832 electrochemical analytical instrument (Chenhua Instrumental Company, Shanghai, China) was connected to the reactor through two Pt electrodes (Rex, 213, China), in which one is as working electrode and the other as counter electrode, and a K2SO4 reference calomel electrode (Rex, 217, China) to record the potential changes. A Type 302 bromide selective electrode was used to measure the change of bromide ion concentration. A microinjector was used for intro-RT ducing potassium bromide solution. A UV757CRT ultraviolet-visible spectrophotometer (Precision & scientific Instrument Company, Shanghai, China) was used to detect the ultraviolet & visible absorption spectra of the mixed solution.
Procedure
A mixed solution containing 4.00 mL of 4.67 × 10−1 M acetone, 2.60 mL of 1.5 × 10−1 M oxalic acid, 7.10 mL of 1.00 M sulfuric acid and 0.90 mL of 1.00 × 10−2 M of Ce(SO4)2 were loaded in a 50 mL water-jacket reactor and mixed well by stirring at 25 ± 0.1 oC. Then, the electrodes were immersed in the mixture solution. After adding 5.40 mL of 6.67 × 10−2 M of potassium bromate, the computer was immediately started to record all signals. At that time a regular steady oscillation profile was observed, this is the initial experiment conditions.
To investigate the effects of double organic substrates, keeping the other conditions as same as has been said above (initial conditions), we adjust one of the volumes to double the organic substrates (acetone or oxalic acid), and then change the volume of sulfuric acid to keep the total volume of 20.0 mL.
Results and Discussion
Effect of oxalic acid and acetone on the oscillating system
Due to no brominated derivative resulting from oxalic acid, oxalic acid could be considered only as a reductant in the FKN mechanism framework rather than as a source of Br−ion. Acetone is a brominatable substrate because it contains an active methylene and its enolization (see Table 3, R10) may be recognized as a rate-determining step in the bromination process. In this study, the effects both oxalic acid and acetone on oscillating system were examined. Results show that the oscillating profile can be observed clearly in the range of 3.75 × 10−3 M-1.35 × 10−1 M for oxalic acid and for acetone from 5.14 × 10−2 M to 4.2 × 10−1 M.
Calculation of bromide ion in the oscillating system
To investigate the effect of the bromination reaction between acetone and bromine, with adjusting the concentration of oxalic acid (or acetone), the concentration of [Br−]baseline was calculated in the oscillating system, in which the concentration of oxalic acid and/or acetone should be adjusted.
Br2 (aq) + 2e− → 2Br− φº = 1.09 V (1)
Based on the Nernst equation, the potential of reaction (1) can be described as follows:

Here, the activity of Br− and Br2 could be approximately regarded as the equilibrium concentration. In terms of this analysis, the eq. 2 can be simplified as follows:

Adding Br− ion (Co) into the oscillating system would remarkably increase the [Br−] of system, at this moment, the changes of [Br2] could be ignored (see Fig. 1). Thus, the change of potential of reaction (1) could be approximately calculated by eq. 4.

The baseline concentration of Br− in the oscillating system can be calculated by adjusting the concentration of organic substrates one by one (see Table 1 and Table 2). Just as shown in Table 1, there is no linear relationship between the concentration of Br− and [(COOH)2], that is, the concentration of oxalic acid is independent of regeneration of Br−. Results in Table 2 show that the [Br−] is directly proportional to the concentration of acetone in the range of 5.14 × 10−2 M-4.20 × 10−1 M. Although this information, that bromination reaction is an important source of Br− ion besides the bromine-hydrolysis reaction, has been noticed by Field and his co-workers, a detail investigation is still required.
Mathematical simulation of B-Z oscillating system
In order to bring the role of bromination reaction in the regeneration of bromide ions to a clear understanding, the bromination reaction between acetone and bromine was used as the removal step of Br2, and the reverse reaction of bromine-hydrolysis reaction (R9) as the source of Br2. According to the new skeleton scheme containing 11 reactions (see Table 3), a modified Oregonator model was obtained (see Table 4), which included three overall processes (Process D to Process F), four independent variables (X, Y, Z, P), and five non-neglected sub-processes (O1 to O5). The rate constants of five sub-processes were defined as k′1, k′2, k′3, k′4, and k′5, separately, where f was a stoichiometric coefficient [13].
In B-Z oscillating system, reaction (O5) should be considered as the overall reaction for bromide ions resulting from various sources, of course, including the bromine-hydrolysis reaction (R9) and the bromination reaction between acetone and bromine (R11). Bromine could be considered as an important intermediate, and converted into bromide ion through the following reactions: a set of non-radical reactions, the bromine-hydrolysis reaction (R9), and an acid-catalyzed bromination reaction. Thereby, bromide ion plays an important role in the control of oscillation behaviors.
The symbols for Oregonator model are as follows: A = BrO3−, B1 = Acetone, B2 = Org (all oxidizable organic species), X = HBrO2, Y = Br−, Z = Ce4+, P = HOBr, in which each symbol stands for the concentration itself. In view of the Debye-Huckel activity coefficient theory of electrolyte solution and the activated complex theory of reaction rate, the B-Z reaction is working at strong ionic strength solution; the activity should be used. Due to the lower concentration of HOBr, HBrO2 and Br− species, however, in this model we use their equilibrium concentration to calculate.

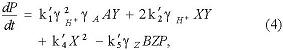
For ease of mathematic simulation, four parameters ε, δ, q, h were introduced, and defined respectively as ε = f(γH+, γZ, γA, k3́, k5́, A, B), δ = f(γH+, γZ, γA, k2́, k3́, k4́, k5́, A, B), q = f(k1́, k2́, k3́, k4́), and f = f(γH+, γZ, γA, k3́, k4́, k5́), to make the above equations being in the dimensionless form. Then, a 4 × 4 system of nonlinear ordinary differential equations was obtained. The calculation was carried out by Mathematica 5.0 on a personal computer to solve the differential equations. Results shown in Fig. 2 indicated that the oscillating behaviors observed both in experiment and in simulation are very similar. That is to say, the proposed model with 11-step skeleton scheme is acceptable.
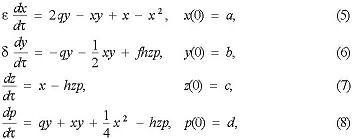
Based on the proposed model, the oscillating behaviors could be explained below: with autocatalytic step (O3) processing, the concentration of Ce4+ ion was increasing to speed up steps (O5) and (O2), and to cause the concentration of [HBrO2] to decrease and the concentration of [Br−] to increase, too. When [Br−] reached a critical value, the consumption amount of HBrO2 in steps (O2) and (O4) would be more than the production amount of HBrO2 in step (O3) to make the step of (O3) slow gradually, and eventually stopped. In this case, step (O1) was found to be dominant in the next cycle (i.e., a new oscillating cycle started), in which R1 could be recognized as a key reaction because its lower rate constant. Similarly, R2 of O2, R3 of O3, and R5 of O4 were also considered to be the rate determining step. Notice that R10 is a key reaction in step (O5), which can offer an intermediate -enolate, to initiate the reaction R11 to regenerate Br− ion. Thereby, the bromination reaction should be considered as an unignored source of Br− ions.
Conclusion
The well-known FKN mechanism is still an important tool in understanding the B-Z oscillating reaction even though some debates in the source of Br− ions have been existed. In the improved model (commonly named Oregonator-like model), although the bromination reaction between enolization of acetone and bromine was mentioned, however, there was no analysis in detail. In the present study, the oscillating system of oxalic acid-acetone-KBrO3-Ce(IV)-H2SO4 was re-examined and an interesting results were obtained, that is, the bromination reaction is also an important source of Br−ions except for the bromine-hydrolysis reaction which was proposed by Field.
Acknowledgements
This work was supported in part by the Project of International Cooperation between China and Ukraine (043-05), the Basic Project of Science and Research of Colleges and Universities of Gansu Province (5001-109), and the Natural Science Foundation of Gansu Province (1010 RJZA015; 096RJZA120).
References
1. Taylo, A. F. Prog. Reac. Kin. Mech., 2002, 27, 247-325. [ Links ]
2. Gao, J.Z. Pak. J. Biol. Sci. 2005, 8, 512-9. [ Links ]
3. Holley, J; Adamatzky, A; Bull, L; Costello, B. De. L. Nano Commun. Networks. 2011, 2, 50-61. [ Links ]
4. Gao, J.Z.; Qu, J.; Yang, W.; Wei, X. X.; Dai, H. X.; Lv, D. Y.; Ren, J.; Chen, H. Amino Acids 2009, 36, 391-7. [ Links ]
5. Field, R. J.; Körös, E.; Noyes, R. M. J. Am. Chem. Soc. 1972, 94, 8649-64. [ Links ]
6. Edelson, D.; Field, R.J.; Noyes, R.M. Int. J. Chem. Kin. 1975, 7, 417-32. [ Links ]
7. Field, R. J.; Noyes, R.M. J. Chem. Phys. 1974, 60, 1877-89. [ Links ]
8. Györgyi, L.; Turányi, T.; Field, R. J. J. Phys. Chem. 1990, 94, 7162-70. [ Links ]
9. Zhabotinsky, A. M.; Rovinsky, A. B. J. Stat. Phys. 1987, 48, 959-75. [ Links ]
10. Nogueira, P.A.; Varela, H.; Faria, R.B. Chem. Phys. Lett. 2012, 530, 137-39. [ Links ]
11. Noszticzius, Z. J. Am. Chem. Soc. 1979, 101, 3660-3. [ Links ]
12. Noszticzius, Z.; Bödiss, J. J. Am. Chem. Soc. 1979, 101, 3177-82. [ Links ]
13. Noszticzius, Z.; Farkas, H. J. Chem. Phys. 1984, 80, 6062-70. [ Links ]
14. Noyes, R. M.; Jwo, J. J. J. Am. Chem. Soc. 1975, 97, 5431-3. [ Links ]
15. Field, R .J.; Boyd, P. M. J. Phys. Chem. 1985, 89, 3707-14. [ Links ]
16. Noszticzius, Z.; Gaspar, V. H.; Foersterling, D. J. Am. Chem. Soc, 1985, 107, 2314-5. [ Links ]
17. Noszticzius, Z.; Farkas, H.; Schelly, Z. A. React. Kinet. Catal. Lett, 1984, 25, 305-11. [ Links ]
18. Hlaváčová, J.; Sevčík, P. Chem. Phys. Lett. 1991, 182, 588-94. [ Links ]
19. Noyes, R. M.; Boyd, P. M. J. Phys. Chem. 1985, 89, 3707-3714. [ Links ]














