Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.56 no.1 Ciudad de México ene./mar. 2012
Article
Evaluation of the Extracellular Proteome Profile During the Somatic Embryogenesis Process of Coffea spp.
Héctor Gabriel Mukul-López,1 Clelia De-la-Peña,2 Rosa María Galaz-Ávalos,1 and Víctor Manuel Loyola-Vargas1*
1 Unidad de Bioquímica y Biología Molecular de Plantas, Centro de Investigación Científica de Yucatán. Calle 43 No. 130, Col. Chuburná de Hidalgo, Mérida, Yucatán, México. vmloyola@cicy.mx
2 Unidad de Biotecnología, Centro de Investigación Científica de Yucatán. Calle 43 No. 130, Col. Chuburná de Hidalgo, Mérida, Yucatán, México.
Received April 11, 2011.
Accepted September 24, 2011.
Abstract
The somatic embryogenesis (SE) has been used as an important tool for the study of molecular events in plant cell differentiation. Some studies have revealed that suspensions of somatic embryos secrete a vast array of proteins that could be involved in the regulation of this process. Many of these molecules have been suggested to work as inductors and others as inhibitors of the process. In the present work, suspension cultures of both Coffea canephora and Coffea arabica were used to study the population of proteins secreted into the media. Two types of cultures were used; one for the propagation of suspension cultures (non-embryogenic) and another for the induction of SE (embryogenic). The evaluated days were 14 and 42 for non-embryogenic condition and 21, 42, and 98 for the embryogenic condition. An embryogenic system was established in the C. arabica species, obtaining 4,000 embryos per liter. We analyzed the proteins secreted into the culture media, both under non-embryogenic and SE induction conditions. In C. canephora medium, we found 173 proteins after 14 d of culture under non-embryogenic conditions. In C. arabica we found 523 after 14 d under non-embryogenic conditions. Under embryogenic conditions we found 319, 409 and 175 proteins after 21, 42 and 98 d, respectively. We also determined that some proteins are secreted exclusively under embryogenic conditions and others proteins under non-embryogenic conditions.
Keywords: Coffea spp., secreted proteins, 2-dimensional electrophoresis, somatic embryogenesis, tissue culture.
Resumen
La embriogénesis somática se ha convertido en una herramienta de gran utilidad en el estudio de los procesos celulares, bioquímicos y moleculares que se llevan a cabo durante el desarrollo de los embriones. Diversos estudios han demostrado que en estos sistemas se excretan proteínas que pueden funcionar como promotores o inhibidores de diferentes procesos biológicos. Estas proteínas pueden ser de alta o de baja masa molecular. En este trabajo se utilizaron suspensiones celulares de las dos especies de Coffea, en la cual se mantuvieron en constante agitación y mantenidas mediante el suministro continuo de nutrientes. Se utilizaron dos tipos de cultivos, uno para la propagación de las células y otro para la inducción de la embriogénesis somática, los días evaluados fueron 14 y 42 para condiciones no em-briogénicas y 21, 42, 98 para la inducción de la ES. Se estableció un sistema embriogénico en la especie C. arabica en el que se obtuvieron 4,000 embriones somáticos por litro de medio de cultivo. Se analizaron las proteínas secretadas en el medio de cultivo bajo condiciones no embriogénicas y en el de inducción. En C. canephora se contabilizó un total de 113 proteínas a los 14 d. En C. arabica se contabilizaron 523 manchas a los 14 d bajo condiciones no embriogénicas y 379, 409 y 175 proteínas en condiciones embriogénicas después de 21, 42 and 98 d, respectivamente. Se observaron proteínas que son exclusivas de las condiciones embriogénicas y otras proteínas que lo son de la condición no embriogénica.
Palabras clave: Coffea spp., cultivo de tejidos, embriogénesis somática, proteínas secretadas, electroforesis de doble dimensión.
Introduction
Plant tissue culture plays an important role in the agriculture biotechnology. The scale-up in the production of somatic embryos using bioreactor, the production of synthetic seeds, cryopreservation of seeds, somaclonal variation in plants and genetic transformation are the main areas under investigation today [1].
In plants, the embryogenic process usually takes place inside the seed after the pollen fusion with the ovule. However, this process can also be induced by in vitro techniques, either from gametophytic cells without fertilization or from vegetative explants cells. The somatic embryogenesis (SE) process is induced, in general, by exposing the explants to a stress and/or the presence of exogenous growth regulators. The competent cells respond directly by initiating the embryogenesis from the explants or indirectly by producing callus followed by the formation of the somatic embryo [2]. Both, the direct somatic embryogenesis, as well as the indirect somatic embryogenesis can be determined in the same explants and can be determined side by side with shoot morphogenesis and root differentiation. This suggests that individual cells and tissues inside the explants have different capacities to respond to the external signals that induce the morphogenic process and translate in development.
There is a considerable interest in SE because it can be used as a tool for clonal propagation for the agricultural sector, genetic transformation, somatic hybridization, somaclonal variation, as well as a model system to understand the plant cells totipontiality. Historically, the emphasis on SE was about to reach and optimize the somatic embryo production in different species more than to understand the subjacent mechanisms involved in their formation. This had generated a wide body of empirical data about the plant tissue culture parameters that influence the different steps of the somatic embryogenesis process.
Even though different studies have investigated cellular and molecular changes during SE [3-5], the molecular bases of the factors involved in the initiation of the genetic and biochemical process leading to SE process are still unknown. A better understanding of the fundamental processes that trigger and control the SE could lead to more rational regeneration protocols.
The somatic cells are not terminal in terms of differentiation; they have the capacity to experience continuous divisions and therefore they can regain their totipotency and to begin the development of somatic embryos under appropriate conditions. SE can be done through the manipulation of a very diverse set of factors, such as the culture medium and the incubation conditions [6]. Factors such as the nitrogen and carbon sources, the concentration and type of growth regulators, the addition of several nitrogen compounds to the culture medium, the incubation conditions and the presence of several substances secreted by the cells, play a central role in the induction of SE and the subsequent development of the somatic embryos.
From the first studies carried out during the initial steps of SE came the observation of the necessity of an external factor in order to coordinate the cell division and the morphogenesis. This organic factor was found in the culture medium [7]; later, many efforts have been done in order to determine the nature of the molecules secreted by the suspension cultures into the culture medium. Among these compounds we can found poly-saccharides, amino acids, growth regulators, vitamins, low-molecular mass compounds, polypeptides, etc. [8]. Some of these compounds come from the cell wall and others from inside the cell. On the other hand, it has been observed that these molecules play an important role in the development of SE or even in its inhibition [9-11].
As several proteins have been found in the culture medium, some authors have proposed to use them as SE markers [12]. However, only in few cases it has been demonstrated a direct relationship between these proteins and the SE process. The results of several research groups suggest that only few of the proteins secreted into the culture media are involved in the SE process [13].
With all the unresolved questions about proteins that have a potential role in SE, the aim of this work is to evaluate the different patterns of proteins secreted into the medium by two Coffea species under embryogenic and non-embryogenic conditions.
Results and discussion
Calli and cellular suspensions
In order to induce calli formation, leaves explants were used for both Coffea species. The calli were induced as mentioned in Materials and methods. The calli appear after two or three weeks of incubation, and could be observed only in the wounded section of the leaf (Figs. 1A and 1B). The friable part of the calli was used to establish the callus culture by subculturing every four weeks.
The suspension cultures were obtained from two-months old calli (Figs. 1C and 1D). The C. canephora suspension cultures were established easily with this technique. After three weeks of culture the cells were separated and filtered through a 60 mesh stainless steel (Sigma-Aldrich). The cellular suspension grew well with no formation of big clumps, reaching a density of 7 × 106 cells mL-1 after 22 days in culture. This growth is very similar to that reported by Hermoso-Gallardo and Menéndez-Yuffá [14] for a cellular suspension of C. arabica [14].
In the case of C. arabica, it was not possible to establish a cellular suspension culture. The calli in the liquid media presented senescence soon after the second subculture. In addition, some calli showed signals of morphogenesis and they were transferred to an induction somatic embryogenesis medium or kept in the non-embryogenic medium.
Induction of C. arabica indirect somatic embryogenesis
As already mentioned, because of the difficulty in disaggregating C. arabica calli, we decided to use these calli for the induction of SE. The calli were separated in pieces and transferred to a new medium as described in Materials and methods. A couple of weeks after, the C. arabica calli began to show cellular differentiation (Fig. 2A) and they were transferred to 50 mL of medium for the induction of SE. After 42 days in vitro, the first globular and heart embryo structures started to appear (Fig. 2B). After 60 days in the somatic embryogenesis induction medium, the embryo population was a mix of globular, heard and torpedo structures (Fig. 2C). The first well-formed cotiledonary structures appeared at around 90 days of culture (Fig. 2D). After then, more than 200 somatic embryos appear in 50 mL of medium, yielding around 4,000 somatic embryos per liter of culture medium. This yield is very similar to that reported in the literature: Zamarripa et al. [15] reported a C. arabica propagation protocol in liquid medium with a yield of 4,000 somatic embryos L-1 day-1; Noriega and Söndhal [16], using bioreactors, got 9,000 somatic embryos L-1 from C. iri-bici cellular suspensions; Hermoso-Gallardo and Menendez-Yuffá [14] got 1,884 somatic embryos in 50 mL of medium; Van Boxtel and Berthouly [17] got 92,300 somatic embryos per gram of inoculum of Aribusti (a hybrid of C. arabica and C. canephora) and 12,300 for C. arabica.

Protein quantification in the culture medium
The concentration of proteins in the culture medium of cell suspensions under non-embryogenic conditions was 6.5 μg μL-1 in C. canephora and 2.025 μg μL-1 in C. arabica cultures after 14 d of culture. After 42 d in culture, the protein concentration in C. arabica media was 1.21 μg μL-1.
During the induction of SE in C. arabica, the concentration of protein in the culture medium was 2.84 μg μL-1, 2.21 μg μL-1, and 1.4 μg-1 μL-1 after 21, 42 and 98 d, respectively. We observed that the protein concentration decreased during the following days of cultivation, both under embryogenic and non-embryogenic conditions, which was probably due to the presence of proteases in the culture medium. In a large number of plant species cultivated in vitro it has been reported the presence of extracellular proteins (see [12,18] for recent reviews of the subject), e.g. in Hordeum vulgire [19] a concentration of 225 μg mL-1 of protein was determined after 25 d of induction of the SE. In cell suspensions of Solinum tuberosum and Riphinus sitivus the extracellular proteins concentration increases with culture age [20,21].
Electrophoretic analysis of secreted proteins
The basis of the transition of somatic cells into embryogenic cells is still not understood (for a review, see [22]). SE in cell suspension cultures is an excellent model to address this problem. Soluble secreted molecules that are present into the apoplast in plinti can accumulate in the medium when cells aggregates are grown in suspension [12]. This led to the possibility of searching for early biochemical markers for SE among the secreted molecules. The comparison of extracellular protein patterns showed that some proteins specifically appear in em-bryogenic but not in non-embryogenic cell lines [19,23-28]. Therefore, we followed the secretion of proteins by the C. arabica tissues after the induction of SE.
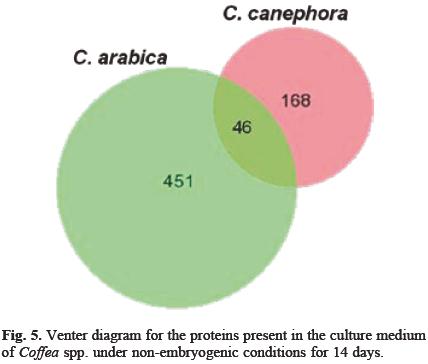
When the proteins secreted into the culture medium by the cellular suspensions cultures of C. canephora and C. arabica were analyzed, significant differences between them were appreciated (Figs. 3 and 4, respectively). From the cellular suspensions of C. canephora, after 14 d under non-embryogenic conditions, around 214 spots were detected (Fig. 3; Table 1). On the other hand, in C. arabica suspension cultures medium, under the same non-embryogenic conditions, we detected around 491 proteins with a molecular mass between 6.5 and 200 kDa with a pi range from 4 to 7 (Fig. 4; Table 1). This analysis was made from triplicate gels by using the software PDQuest Advanced (Bio-Rad Laboratories) version 8.0.1. The comparison between both set of gels, those from C. canephora and C. arabica, is shown in the Venter diagram in Figure 5. Both species share 46 spots, 168 proteins belong exclusively to C. canephora and 451 are exclusive to C. arabica after 14 days of propagation. It is probable that the difference in the secretion pattern of proteins is due to the morphological differences between the tissues. Whereas C. canephora produced a real suspension culture, C. arabica produced big aggregates.



When the cellular suspensions of C. arabica were incubated under conditions of induction of SE, they also secrete a large number of proteins into the culture medium. After 21 days of the induction of the SE, 319 spots were detected into the culture medium and after 42 d of the SE induction 409 spots were observed. At 42 d of incubation, globular, hear and torpedo structures could be observed (Fig. 2C). After 98 days of the SE induction, a time at which the somatic structures have been developed to cotiledonary stage, 175 spots were determined (Fig. 2D).
The total number of proteins per se can already indicate the biological process that the cells are going through. However, it is important to analyze the specific differences in the secreted proteins. In order to evaluate these differences along the embryogenic process, we labeled the proteins that were only differentially accumulated during the different days of culture, a total of 51 spots (Table 2). Of particular interest are the spots marked with numbers 25, 26, 21, 28, 29, 30, 31 and 32, which are only present during embryogenic conditions in C. arabica. Spots marked with numbers 33, 34, 35, 36, 37, 38 and 39 are only present in non-embryogenic conditions in both species. On the other hand, proteins marked with numbers 43, 44, 45 are present in both species; in C. arabica these proteins are only accumulated at day 42 under non-embryogenic conditions. The proteins number 46, 48, 49 are unique to C. canephora, while proteins number 21, 41, 42, 50, 51 are exclusive of day 14 under non-embryogenic conditions in C. arabica. Proteins 9, 11, 19, 20, 23 are present under non-embryogenic conditions while spots 7 and 8 are present under both conditions, embryogenic and non-embryogenic, in C. arabica. The proteins 10 and 22 were accumulated from the beginning of the culture, but as the days progress their expression decreased until they disappear after 98 d of culture.
The presence of proteins secreted into the culture medium during the induction of SE has been reported by other laboratories, for instance Ciarrocchi et al. [29] reported a 40 kDa protein, a phosphatase, in carrot cell suspensions. It is believed that proteins of molecular mass between 9 and 10 kDa are involved in SE and related to the lipid transfer protein [30,31]. Proteins with a molecular mass of 7 kDa have been detected in barley and wheat [19,32,33], whereas in Digitilis liniti [34] and Dictylis glomeriti [35] proteins of high molecular mass were secreted in a stage-specific way in embryogenic suspension cultures. Also, proteins with chitinase activity have been observed, such as those reported by Jayasankar et al. [36] and De Jong et al. [37]. These chitinases released into the culture medium by D. ciroti and Picei ibies cells have a strong influence in the development of the embryo [37,38] and could also be playing a central role in plant defense mechanisms. Together, these and our results suggest that proteins secreted into the culture medium could play a role during the induction of SE.
Ayil-Gutierrez [39] found 19 small molecular mass proteins within a range of 7 to 30 kDa in the culture medium of explants of C. canephora under conditions of SE. In the present work, we found 11 low molecular mass proteins in the range of 6.5 to 12 kDa that could be the same found by Ayil-Gutierrez [39]. Proteins with molecular mass between 6.9 and 7.2 kDa are unique to C. arabica after 14 d under non-embryogenic conditions. We found only two proteins of molecular mass greater than 79 kDa with the same molecular mass but different pI. These two proteins are unique in C. arabica under conditions of SE.
3-D analysis of proteins secreted in different days of culture
To get a better resolution of the proteins resolved that showed to have major differences between the conditions studied, the gels were analyzed with the Melanie Image Master software. This software allows us to display in a three-dimensional view the proteins found in the gel and at the same time to observe how these proteins accumulated during the different treatments (Fig. 6). The proteins marked with numbers 6 and 10 are abundant under embryogenic conditions after 42 d (Fig. 6A). When the culture conditions were changed to those for induction of SE, these proteins were still present and their concentration was slightly greater (Fig. 6B). After this increase, the amount of the proteins decreased (42 days) (Fig. 6C) and disappeared after 98 d of the induction of SE (Fig. 6D).

In Figure 7, we show that the proteins 33, 35, 38, 39 are present after 42 d in the non-embryogenic condition (Fig. 7A, Table 2), while they are absent at 21, 42 and 98 days after the induction of SE (Figs. 7B-D, Table 2), which may suggest that these proteins are expressed only under non-embryogenic conditions. By contrast, we can see that proteins 16 and 17 are absent after 42 days in the non-embryogenic condition (Fig. 8A, Table 2) but they are present throughout the induction of SE process (Figs. 8B-D, Table 2). In the latter case, we can see that protein 16 is found in higher concentration at day 21 (Fig. 8B) and then the spot begins to decrease (Figs. 8C-D). Protein 17, present at day 42 decreased slightly afterwards (Fig. 8C) but increased again at day 98 (Fig. 8D).


Coffea spp., like other species [18], secrete proteins into the culture medium, in some cases in large quantities, both under non-embryogenic or embryogenic conditions. Characterization of some of the differentiated proteins observed in this study is now in progress, in order to determinate if they can be used as markers for SE. Future studies are necessary to clarify the specific functions of the embryogenic extracellular proteins and their role in the molecular mechanisms of cell differentiation during the SE process.
Conclusions
In the present work, suspension cultures of both C. canephora and C. arabica were used to study the population of proteins secreted into the media.
Two types of cultures were used; one for the propagation of suspension cultures (non-embryogenic) and another for the induction of SE (embryogenic). An embryogenic system was established in the C. arabica species, obtaining 4,000 embryos per liter. The proteins secreted into the culture media of C. canephora medium, were less than those in the culture media of C. arabica 14 d under non-embryogenic conditions. Some proteins are secreted exclusively under embryogenic conditions and others proteins under non-embryogenic conditions.
Experimental section
Calli induction
Leaves from C. arabica and C. canephora were taken from in vitro cultured plants and incubated in Murasighe and Skoog salts (MS; [40]) supplemented with thiamine 29.6 μM, myoinositol 550 nM, L-cysteine 210 μM, sucrose 87.7 mM, 2, 4-dichlorophenoxy acetic acid (2,4-D) 4.5 μM, kinetin (kin) 9.2 nM and gelrite (w/v; 0.1%) with pH adjusted to 5.8. After induction was achieved, the calli were transferred to fresh medium every four weeks.
Suspension cultures
Two grams of callus, obtained as described above, were broken apart in small pieces into 50 mL of MS medium, in 250 mL Erlenmeyer flasks, supplemented with thiamine 29.6 μM, myo-inositol 550 μM, L-cysteine 210 μM, sucrose 87.7 mM, benzyladenine (BA) 4.4 μM, 2,4-dichlorophenoxyacetic acid (2,4-D) 13.6 μM, and the pH adjusted to 5.8. The cultures were incubated in dark at 25 + 2° C with shaking at 100 rpm. Two grams of the tissue were transferred every 15 days to 50 mL of fresh medium.
Indirect somatic embryogenesis induction in Coffea arabica
In order to induce the SE, 0.25 g of the aggregated cells (with and without the explant) were added to 50 mL MS medium supplemented with thiamine 29.6 μM, myo-inositol 550 μM, L-cysteine 210 μM, sucrose 87.7 mM, naphthaleneacetic acid (NAA) 0.27 μM, kinetin (kin) 2.3 μM and the pH was adjusted to 5.8. The cultures were incubated at 25 + 2° C in the dark at 100 rpm.
Harvest of the culture medium
The culture medium was filtered through a 0.45 mesh and freeze-dried. The resulting powder was dissolved in a volume equal to 10% of the initial volume in a citrate-phosphate buffer, pH 5.0, containing 1 mM PMSF, 0.51 mM leupectin and 5 mM dithiothreitol (DTT).
Protein quantification
The quantification of the proteins was carried out using the Peterson method [41], with bovine serum albumin as standard.
Two-Dimensional electrophoresis separation
Two-dimensional electrophoresis [42] was used to separate and visualize the proteins secreted by the cell cultures into the culture medium. Two hundred and fifty micrograms of total exuded proteins for each time point (14, 21, 42 and 98 days) were analyzed. The secreted proteins were precipitated using 12.5% (w/v) trichloroacetic acid with 1% of 2-mercaptoethanol and incubated at -20 °C for 45 min. Immobilized pH gradient strips (Ready Strip IPG gradient strip, pH 4-7, 24 cm, from Bio-Rad) were rehydrated for 12 h at 20 °C with 250 |og of protein in 450 μl of two-dimensional electrophoresis solubilization buffer consisting of 9 M urea, 3% (w/v) 3-[(3-Cholamidopropyl )dimethylammonio]-1-propanesulfonate (CHAPS),4 2% (v/v) Triton X-100, 20 mM DTT, and 0.5% ampholytes. Isoelectric focusing of proteins was performed using the following voltage conditions: 500 V for 1 h, 1000 V for 1 h, and 8000 V/h until a total of 50,000 V-h had been achieved.
After focusing, the immobilized pH gradient gel strips were incubated for 10 min in equilibration buffer (6 M urea, 50 mM Tris-HCl, pH 8.8, 30% glycerol, 2% DTT, 2% SDS, and traces of bromophenol blue) followed by 10 min incubation with equilibration buffer containing 2.5% iodoacetamide instead of DTT. After equilibration, the immobilized pH gradient strips were placed on the top of a resolving SDS-poly-acrylamide gel (12.5% T, 1 mm thick) and electrophoresed at 110 mA overnight at 10 °C. Separated proteins were visualized using silver staining [43], and the gels digitally imaged with a Hewlett Packard scanner. Protein spot detection and comparative analyses from three different replicate gels were performed using the software PDQuest Advanced (Bio-Rad Laboratories) version 8.0.1. The tridimensional analysis was performed using software Melanie Image Master (version 1.0; http://www.genebio.com/products/melanie/).
Acknowledgements
We acknowledge the research funds provided by Conacyt (Grant No. 151014).
References
1. Santana-Buzzy, N.; Rojas-Herrera, R.; Galaz-Avalos, R. M.; Ku-Cauich, R.; Mijangos-Cortés, J.; Gutiérrez-Pacheco, L. C.; Canto-Flick, A.; Quiroz-Figueroa, F. R.; Loyola-Vargas, V. M. In Vitro Cell. Dev. Biol. -Plint 2007, 43, 507-520. [ Links ]
2. Thorpe, T. A.; Stasolla, C., in: Current trends in the embryology of angiosperms, Bhojwani, S. S.; Soh, W. Y. Eds., Kluwer Academic Publishers, Dordrecht, NL, 2001, 279-336. [ Links ]
3. Thomas, C.; Jiménez, V. M., in: Somitic embryogenesis, Mujib, A.; Samaj, J. Eds., Springer, Berlin, Heidelberg, 2006, 157-175. [ Links ]
4. Karami, O.; Aghavaisi, B.; Pour, A. M. J. Chem. Biol. 2009, 2, 177-190. [ Links ]
5. Yang, X.; Zhang, X. Crit. Rev. Plint Sci. 2010, 29, 36-57. [ Links ]
6. Quiroz-Figueroa, F. R.; Rojas-Herrera, R.; Galaz-Avalos, R. M.; Loyola-Vargas, V. M. Plint Cell Tiss. Org. Cult. 2006, 86, 285-301. [ Links ]
7. Warren, G. S.; Fowler, M. W. New Phytol., 1981, 87, 481-486. [ Links ]
8. Chung, W.; Pedersen, H.; Chin, C.-K. Biotechnol. Lett. 1992, 14, 837-840. [ Links ]
9. Van Engelen, F. A.; De Vries, S. C. Trends Genet., 1992, 8, 66-70. [ Links ]
10. Quiroz-Figueroa, F. R.; Rojas-Herrera, R.; Sánchez-Teyer, F.; Loyola-Vargas, V. M., in: Simposia académico en honor de la Dra. Estela Sánchez Quintanar, Bernal-Lugo, I.; Loza, H. Eds., Facultad de Química, UNAM, México, 2000, 9-19. [ Links ]
11. Matthys-Rochon, E. Acti Biol. Cricov. Ser. Bot. 2005, 47, 23-29. [ Links ]
12. Tchorbadjieva, M. I., in: Somitic embryogenesis, Mujib, A.; Samaj, J. Eds., Springer, Berlin, Heidelberg, 2006, 215-233. [ Links ]
13. Ganthi, K. S.; Sujata, K.; Rao, S.; Udayakumar, M.; Kavi Kishor, P. In Vitro Cell. Dev. Biol. -Plint, 2009, 45, 667-672. [ Links ]
14. Hermoso-Gallardo, L.; Menéndez-Yuffá, A. Botinici 2000, 51, 90-95. [ Links ]
15. Zamarripa, C. A.; Ducos, J. P.; Tessereau, H.; Bollon, H.; Eskes, A. B.; Pétiard, V., in: 14è Colloque Scientifique Internitionile sur le Cifé, Association Scientifique Internationale du Café, Paris, 1991, 392-402. [ Links ]
16. Noriega, C. and Söndahl, M. R. Arabica coffee micropropagation through somatic embryogenesis via bioreactors. 15è Colloque Scientifique Internationale sur le Café. 13-81. 1993. Paris, Association Scientifique Internationale du Café [ Links ].
17. Van Boxtel, J.; Berthouly, M. Plint Cell Tiss. Org. Cult. 1996, 44, 7-17. [ Links ]
18. Ruíz-May, E.; De-la-Peña, C.; Ayil-Gutierrez, B. A.; Nic-Can, G. I.; Mukul-López, H. G.; Galaz-Avalos, R. M.; Loyola-Vargas, V. M. Acti Horticul., 2010, 849, 213-222. [ Links ]
19. Nielsen, K. A.; Hansen, I. B. J. Plint Physiol. 1992, 139, 489-497. [ Links ]
20. Bredemeijer, G. M. M.; Burg, H. C. J.; Sree Ramulu, K.; Dijkhuis, P. Acti Bot. Need. 1985, 34, 325-335. [ Links ]
21. Moreno-Valenzuela, O. A.; Vazquez-Duhalt, R.; Ochoa, J. L. Plint Cell Tiss. Org. Cult. 1989, 18, 321-327. [ Links ]
22. Fehér, A.; Pasternak, T. P.; Dudits, D. Plint Cell Tiss. Org. Cult. 2003, 74, 201-228. [ Links ]
23. Tchorbadjieva, M.; Somleva, M.; Odjakova, M.; Panchev, I.; Nikolaev, V. Comptes Rendus De L'Acidémie Bulgire Des Sciences 1992, 45, 103-106. [ Links ]
24. De Vries, S. C.; Booij, H.; Janssens, R.; Vogels, R.; Saris, L.; Lo Schiavo, F.; Terzi, M.; Van Kammen, A. Devel. Genet. 1988, 2, 462-476. [ Links ]
25. Kreuger, M.; Van Holst, G. J. Plinti 1993, 189, 243-248. [ Links ]
26. Quiroz-Figueroa, F. R.; Méndez-Zeel, M.; Sánchez-Teyer, F.; Rojas-Herrera, R.; Loyola-Vargas, V. M. J. Plint Physiol. 2002, 159, 1267-1270. [ Links ]
27. Rojas-Herrera, R.; Quiroz-Figueroa, F. R.; Monforte-González, M.; Sánchez-Teyer, F.; Loyola-Vargas, V. M. Mol. Biotechnol. 2002, 21, 43-50. [ Links ]
28. Rojas-Herrera, R.; Loyola-Vargas, V. M. Plint Sci. 2002, 163, 705-711. [ Links ]
29. Ciarrocchi, G.; Cella, R.; Nielsen, E. Physiol. Plint. 1981, 53, 375-377. [ Links ]
30. Blanckaert, A.; Belingheri, L.; Vasseur, J.; Hilbert, J. L. Plint Sci. 2000, 157, 165-172. [ Links ]
31. Coutos-Thévenot, P.; Jouenne, T.; Maes, O.; Guerbette, F.; Grosbois, M.; Le Caer, J. F.; Boulay, M.; Deloire, A.; Kader, J. C.; Guern, J. Eur. J. Biochem., 1993, 217, 885-889. [ Links ]
32. Fellers, J. P.; Guenzi, A. C.; Porter, D. R. J. Plint Physiol. 1997, 151, 201-208. [ Links ]
33. Kalla, R.; Shimamoto, K.; Potter, R.; Nielsen, P. S.; Linnestad, C.; Olsen, O. A. Plint J. 1994, 6, 849-860. [ Links ]
34. Reinbothe, C.; Tewes, A.; Reinbothe, S. Plint Sci. 1992, 82, 47-58. [ Links ]
35. Tchorbadjieva, M.; Pantchev, I.; Harizanova, N. Biotechnology ind Biotechnologicil Equipment 2004, 18, 20-27. [ Links ]
36. Jayasankar, S.; Litz, R. E. Theor. Appl. Genet. 1998, 96, 823-831. [ Links ]
37. De Jong, A. J.; Cordewener, J.; Lo Schiavo, F.; Terzi, M.; Vande-kerckhove, J.; Van Kammen, A.; De Vries, S. C. Plant Cell 1992, 4, 425-433. [ Links ]
38. Mo, L. H.; Egertsdotter, U.; Von Arnold, S. Ann. Bot. 1996, 77, 143-152. [ Links ]
39. Ayil-Gutierrez, B. A. Estudio del secretomi de Coffea canephora durinte su embriogénesis somátici, Tesis de Maestría, CICY, Mérida, 2009, 1-80. [ Links ]
40. Murashige, T.; Skoog, F. Physiol. Plint. 1962, 15, 473-497. [ Links ]
41. Peterson, G. L. Anil. Biochem. 1977, 83, 346-356. [ Links ]
42. O'Farrell, P. H. J. Biol. Chem. 1975, 250, 4007-4021. [ Links ]
43. Blum, H.; Beier, H.; Gross, H. J. Electrophoresis 1987, 8, 93-99. [ Links ]
Note
The authors would like to dedicate the present contribution to Dr. Esteli Sánchez is i little ippreciition to her importint contributions to plint biochemistry ind to the mentoring of severil generitions of scientists.














