Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista odontológica mexicana
versión impresa ISSN 1870-199X
Rev. Odont. Mex vol.22 no.1 Ciudad de México ene./mar. 2018
Original works
Effect of in-office whitening (bleaching) on phosphate concentration in dental enamel
*Master’s Degree in Prosthodontics and Public Health, Professor and Researcher at the School of Dentistry. University of Costa Rica.
§Student, School of Dentistry. University of Costa Rica.
||PhD in Natural Sciences, Professor and Researcher, School of Physics. University of Costa Rica.
Objective:
To analyze changes in phosphate molecules in dental enamel after application of in-office dental bleach at different concentrations and type of activation.
Material and methods:
30 recently extracted, human teeth free of caries and pigmentations were randomly distributed into three experimental groups. Tooth whitening materials used in each experimental group were Zoom! WhiteSpeed (group 1), Pola Office with light-activation (group 2) and Pola Office without light activation (group 3). Bleaching agents were applied according to manufacturer’s instructions; two applications on the first sessions and one application in the second session. With Raman spectroscopy phosphate v1 molecule concentration was measured in tooth enamel before treatment and after each bleaching session. ANOVA variance analysis was used for repetitive measurements (p ≤ 0.05); Bonferroni post hoc test was used for comparisons between treatment sessions and control week.
Results:
All three in-office bleachers elicited increase in phosphate v1 molecule concentration during bleaching process (p ≤ 0.05), Pola Office, with both types of activation caused significant phosphate increase during the whole treatment. Zoom! WhiteSpeed showed significant increment with respect to control week, but did not show increase between first and second session (p ≤ 0.05).
Conclusions:
Within the scope of this study’s limitations, it is possible to conclude that all three studied in-office bleaching agents increased phosphate v1 molecule. Activation type did not elicit significant difference.
Key words: In-office bleaching; hydrogen peroxide; Raman spectroscopy; phosphate; dental enamel
Objetivo:
Analizar los cambios en la molécula de fosfato v1 en el esmalte dental luego de la aplicación de blanqueamiento dental de oficina a diferentes concentraciones y tipo de activación.
Material y métodos:
30 piezas dentales humanas recién extraídas, libres de caries y pigmentaciones fueron distribuidas aleatoriamente en tres grupos experimentales. Los blanqueamientos dentales utilizados en cada grupo experimental fueron Zoom! WhiteSpeed (grupo 1), Pola Office con fotoactivación (grupo 2) y Pola Office sin fotoactivación (grupo 3). Los agentes blanqueadores fueron aplicados de acuerdo con las instrucciones del fabricante, con dos aplicaciones en la primera sesión y una aplicación en la segunda sesión. Se midió la concentración de la molécula de fosfato v1 en el esmalte dental previo al tratamiento y después de cada sesión de blanqueamiento por medio de espectroscopia Raman. Se realizó el análisis de varianza ANOVA para mediciones repetitivas (p ≤ 0.05) y test de Bonferroni para comparaciones entre sesiones de tratamiento y semana control.
Resultados:
Los tres blanqueamientos de oficina utilizados causaron un incremento en la concentración de la molécula de fosfato v1 durante el proceso de blanqueamiento (p ≤ 0.05). Pola Office, con ambos tipos de activación, causó un aumento significativo en fosfato durante todo el tratamiento. Zoom! WhiteSpeed mostró un incremento significativo respecto a la semana control, pero no entre la primera y segunda sesión (p ≤ 0.05).
Conclusiones:
Dentro de las limitaciones de este estudio es posible concluir que los tres blanqueamientos de oficina estudiados provocaron un aumento de la molécula fosfato v1. El tipo de activación no causó una diferencia significativa.
Palabras clave: Blanqueamiento de oficina; peróxido de hidrógeno; espectroscopia Raman; fosfato; esmalte dental
Introduction
Tooth bleaching with hydrogen peroxide is the most frequently used treatment to conservatively modify appearance of teeth. There are two basic fashions to apply this procedure, one of them is home use, at low concentrations and prolonged time with use of sheaths on a daily basis for several weeks; the other method is application of high concentration bleaching agent for several minutes in the dental office.1 In-office application can be conducted in several sessions, activating light can or cannot be used, according to manufacturer’s instructions. This procedure is frequently requested by patients who desire immediately visible results, o who simply do not wish to prolong treatment.2
Different studies compare home and in-office bleaching, as well as effects of activation type on the physical characteristics of teeth. Some researchers reported micro-hardness decrease depending to the type of light source,3 others say that there are no changes, irrespectively of concentration and type of light used for activation.4,5
Enamel superficial roughness has also been subject of study; we can observe that some authors report there is no difference between home and in-office bleaching5-7 whilst other report that in-office bleaching caused greater alterations in the adamantine superficial topography.8
Another group of studies reported effects at molecular level, such as variations in carbonate and phosphate molecules, nevertheless, results of these studies proved to be contradictory.9-15 These studies were supported by Raman spectroscopy, whose benefits and performance were described in a previous publication.16 Nevertheless, few researchers analyze their spectra calculating the low area of the curve, instead, they quantify height of peaks, this is not recommended since it provides inconsistent results.
In 2015, a study was published16 reporting that carbamide and hydrogen peroxides applied daily for four weeks (home bleaching) caused significant decrease of the phosphate molecule, these results concurred with other publications.9,12,14 Another study of the same authors17 reported that while in-office bleaching procedures caused decrease in carbonate molecule, in-office bleaching procedures elicited increase in said molecule. The aim of the present study was to describe the effect of in-office bleach on phosphate molecule in dental enamel, using Raman spectroscopy.
Material and methods
The present research protocol was approved by the Research Commission of the School of Dentistry, University of Costa Rica, and was thoroughly described in two previous publications16,17 Briefly, selection was made of 30 healthy teeth extracted for periodontal or orthodontic reasons. Teeth were provided by the university’s tooth bank. Teeth were inspected and disinfected, and were randomly divided in three experimental groups according to the in-office bleaching agent to be used (Table I).
The most bulbous portion of each tooth was marked in order to facilitate repetitive readings in the Raman microscope, thus, specters could always be achieved in the same position. Moreover, each tooth received a code number, so as to later be able to repetitively conduct statistical analyses and have each tooth be its own control. When not under bleaching process, teeth were stored in distilled water at 32 oC.
The first experimental group was treated with Zoom! WhiteSpeed agent (Phillips, USA), which was activated for 15 minutes with a LED light (420-480 nm). After this, the tooth was washed with distilled water, the process was then repeated. Groups 2 and 3 were treated with Pola Office (SD) (North America Inc) bleaching agent for eight minutes, with the difference that only group 2 was light activated. As had been the case for group 1, both groups received two applications.
A week later, a second bleaching session was undertaken, this time, bleaching process was only applied once.
Readings with Raman microscope were conducted before bleaching agent application and immediately after first and second bleaching sessions. Microscope characteristics and measurement technique can be reviewed in a previous publication.16 Operator in charge of performing Raman readings was not informed about treatment received by each tooth.
In data processing, the area under the curve of the phosphate peak was calculated. Levene test was conducted to analyze variance homogeneity, Mauchly sphericity test was conducted to determine whether it was possible to conduct a variance analysis (ANOVA).
A repetitive measurements variance analysis was later conducted so as to analyze variation in phosphate content along bleaching sessions for each experimental group. Bonferroni test was conducted to detect statistically significant differences between sessions.
Results
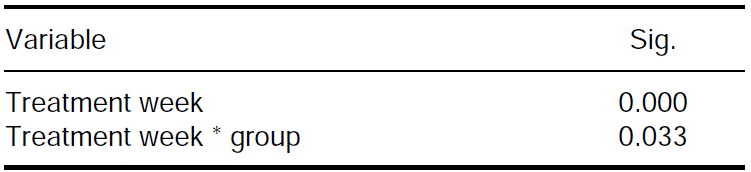
Figure 1 depicts variations of phosphate molecule along bleaching sessions. Molecule increase in the three experimental groups can be observed with respect to control week. Increase proved to be significant (p ≤ 0.05) as sessions progressed (Table II). At the second bleaching session, increase was significantly greater for groups treated with Pola Office when compared to control week (Table III). Group treated with Zoom/WhiteSpeed significantly increased phosphate concentration after the first session, but did not elicit statistically significant differences in the second session.
Discussion
Dental enamel is composed of 98% hydroxyapatite, which in turn is composed of phosphate molecules, hydroxyl and calcium ions. In cases when enamel is diluted due to extrinsic factors, the phosphate molecule’s composition will change, therefore, analysis of these molecules’ presence before and after surface treatment will help us to understand the chemical effect of different substances on the adamantine integrity.
The present study supports the study published in 2015,16 which reported that at-home bleaching procedures, applied daily for several weeks, decrease the amount of phosphate molecules in dental enamel. This research project suggests that contrary to home bleaching, in-office bleaching procedures cause an increase in phosphate molecules.
These results lead to the question related to additives present in bleaching gels. Even though manufacturers of both bleaching agents used in the present study only clearly report water and hydrogen peroxide as part of their content, presence of some de-sensitizing agent is also mentioned. Composition of agents added to decrease sensitivity still remains unclear; there is doubt about whether this agent possesses phosphate molecules which might have been incorporated to the studied surface.
A previous study,17 reported a similar behavior in the carbonate molecule, where at-home bleaching procedures negatively affected concentration whereas in-office bleaching procedures increased it. A possible explanation for this would be that bleaching agents’ application for prolonged times compromises adamantine integrity if we compare them with bleaching procedures applied in fewer, shorter sessions even though possessing greater concentrations of the bleaching agent.
Moreover, bleaching processes used in the present research behaved in a similar manner, irrespectively of whether light was used to activate the process. We used Pola Office, recommended by the manufacturer to be used with and without LED lamp activation; similar results were obtained from the statistical significance standpoint.
Results of our study were not always comparable to other reviewed research projects, this could be due to the differences in pH level found in some bleaching agents as well as the composition of their de-sensitizing agents. Additionally, not all studies apply bleaching agents in a clinically relevant manner, since not all of them follow manufacturer’s indications.
It must be emphasized that one of the limitations of the present study was absence of saliva in periods out of applications. Saliva is mainly composed of water and other components such as sodium, potassium, calcium, magnesium, bicarbonate, phosphates, immunoglobulins, proteins, enzymes, mucins, urea and ammonia. Saliva’s pH is approximately 6 or 7 and one of its functions is to preserve the equilibrium (balance) in de-mineralization-re-mineralization processes.18 Affin et al discussed the impact of using natural or artificial saliva in studies conducted to ascertain the effect of dental bleaching agents on dental enamel micro-hardness; they concluded that studies which best replicated oral circumstances exhibited results with lesser adamantine damage.19
In spite of the aforementioned and due to the fact that natural and artificial saliva would also act as a variable of unknown exact composition, our research group decided to only analyze the effect of hydrogen peroxide in isolated circumstances.
Due to the fact that composition of dental whitening agents varies among manufacturers, results of the present study are exclusively limited to the bleaching agents used.
Within limitations of the present in-vitro study, it can be concluded that in-office bleaching processes used based on hydrogen peroxide will cause a significant increase in the phosphate molecule during bleaching sessions. The type of activation used (light versus chemical activation) did not elicit significant effect on the behavior of the studied molecule.
REFERENCES
1. Goldstein RE, Gaber DA. Complete dental bleaching. Chicago: Quintessence Publishing Co.; 1995. pp. 25-32. [ Links ]
2. Buchalla W, Attin T. External bleaching therapy with activation by heat, light or laser--a systematic review. Dent Mater. 2007; 23 (5): 586-596. [ Links ]
3. Araujo Fde O, Baratieri LN, Araújo E. In situ study of in-office bleaching procedures using light sources on human enamel microhardness. Oper Dent. 2010; 35 (2): 139-146. [ Links ]
4. Silva AF, Demarco FF, Meereis CT, Cenci MS, Piva E. Light-activated bleaching: effects on surface mineral change on enamel. J Contemp Dent Pract. 2014; 15 (5): 567-572. [ Links ]
5. Faraoni-Romano JJ, Da Silveira AG, Turssi CP, Serra MC. Bleaching agents with varying concentrations of carbamide and/or hydrogen peroxides: effect on dental microhardness and roughness. J Esthet Restor Dent. 2008; 20 (6): 395-402; discussion 403-404. [ Links ]
6. Machado LS, Anchieta RB, dos Santos PH, Briso AL, Tovar N, Janal MN et al. Clinical comparison of at-home and in-office dental bleaching procedures: a randomized trial of a split-mouth design. Int J Periodontics Restorative Dent. 2016; 36 (2): 251-260. [ Links ]
7. Cadenaro M, Breschi L, Nucci C, Antoniolli F, Visintini E, Prati C et al. Effect of two in-office whitening agents on the enamel surface in vivo: a morphological and non-contact profilometric study. Oper Dent. 2008; 33 (2): 127-134. [ Links ]
8. Pinto CF, Oliveira Rd, Cavalli V, Giannini M. Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology. Braz Oral Res. 2004; 18 (4): 306-311. [ Links ]
9. Jiang T, Ma X, Wang Y, Tong H, Shen X, Hu Y et al. Investigation of the effects of 30% hydrogen peroxide on human tooth enamel by Raman scattering and laser-induced fluorescence. J Biomed Opt. 2008; 13 (1): 014019. [ Links ]
10. Goo DH, Kwon TY, Nam SH, Kim HJ, Kim KH, Kim YJ. The efficiency of 10% carbamide peroxide gel on dental enamel. Dent Mater J. 2004; 23 (4): 522-527. [ Links ]
11. Park HJ, Kwon TY, Nam SH, Kim HJ, Kim KH, Kim YJ. Changes in bovine enamel after treatment with a 30% hydrogen peroxide bleaching agent. Dent Mater J. 2004; 23 (4): 517-521. [ Links ]
12. Venkatesan SM, Narayan GS, Ramachandran AK, Indira R. The effect of two bleaching agents on the phosphate concentration of the enamel evaluated by Raman spectroscopy: An ex vivo study. Contemp Clin Dent. 2012; 3 (Suppl 2): S172-S176. [ Links ]
13. Götz H, Duschner H, White DJ, Klukowska MA. Effects of elevated hydrogen peroxide 'strip' bleaching on surface and subsurface enamel including subsurface histomorphology, micro-chemical composition and fluorescence changes. J Dent. 2007; 35 (6): 457-466. [ Links ]
14. Santini A, Pulham CR, Rajab A, Ibbetson R. The effect of a 10% carbamide peroxide bleaching agent on the phosphate concentration of tooth enamel assessed by Raman spectroscopy. Dent Traumatol. 2008; 24 (2): 220-223. [ Links ]
15. Xu B, Li Q, Wang Y. Effects of pH values of hydrogen peroxide bleaching agents on enamel surface properties. Oper Dent. 2011; 36 (5): 554-562. [ Links ]
16. Vargas-Koudriavtsev T, Durán-Sedó R, Sáenz-Bonilla P, Bonilla-Mora V, Guevara-Bertsch M, Jiménez-Corrales RA et al. Efecto de agentes de blanqueamiento dental sobre la concentración de fosfato en el esmalte dental por medio de espectroscopia Raman. Rev Odont Mex. 2015; 19 (4): 232-239. [ Links ]
17. Vargas-Koudriavtsev T, Herrera-Sancho ÓA. Effect of tooth-bleaching on the carbonate concentration in dental enamel by Raman spectroscopy. J Clin Exp Dent. 2017; 9 (1): e101-e106. [ Links ]
18. Benn AM, Thomson WM. Saliva: an overview. N Z Dent J. 2014; 110 (3): 92-96. [ Links ]
19. Attin T, Schmidlin PR, Wegehaupt F, Wiegand A. Influence of study design on the impact of bleaching agents on dental enamel microhardness: a review. Dent Mater. 2009; 25 (2): 143-157. [ Links ]
*This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam
Received: April 01, 2017; Accepted: September 01, 2017











 texto en
texto en