Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista odontológica mexicana
Print version ISSN 1870-199X
Rev. Odont. Mex vol.17 n.4 Ciudad de México Oct./Dec. 2013
Original research
Influence exerted by a xylitol and fluoride based mouthwash on the in vitro enamel remineralization of primary teeth
Cinthya Cobos Ortega,* Emilia Valenzuela Espinoza,§ Miguel Ángel AraizaII
* Professor and Pedodontics Specialist, Graduate and Research Division, National School of Dentistry, National University of Mexico (UNAM).
§ Professor, Pedodontics Specialty, Graduate and Research Division National School of Dentistry, National University of Mexico (UNAM).
II Professor, Dental Materials Laboratory, Graduate and Research Division, National School of Dentistry, National University of Mexico (UNAM).
ABSTRACT
The purpose of the present study was to assess the effectiveness on primary teeth of a fluoride and xylitol based mouthwash. 40 caries-free teeth were used. 35% phosphoric acid was applied during 20 seconds. Teeth were then immersed in the mouthwash for 0, 15, 30, 45 and 60 days. 150-250μm longitudinal slices were taken of each sample. Re-mineralization was assessed according to bi-refringence observed after applying Thoulet solution (1.47 IR). Assessment was conducted under polarized light in a photo-microscope. At 15 days, a mean of 0.444 (± 0.527) was observed. After 30 days the observed mean was 0.778 (± 0.441). At 45 days, observed mean was 1.444 (± 0.527), and at 60 days, observed mean was 1.47 (± 0.483). Variance analysis established statistically significant differences among groups (p < 0.001) as well as when comparisons among groups were established (p < 0.05). After conducting the aforementioned tests it could be concluded that the employed mouthwash exerted a slight re-mineralizing effect upon the enamel of treated teeth.
Key words: Fluoride, xylitol, enamel, re-mineralization, primary teeth, polarized light, bi-refringence.
INTRODUCTION
In general terms, products used for oral hygiene contain anti-microbial effect substances which can decrease caries incidence through a mechanism of plaque build-up control, suppressing thus cariogenic species or through the inhibition of the bacterial mechanism.1
Since 1970 changes in the mean index of caries have been spectacular. In this field, fluoride plays a main role, through the action exerted by three mechanisms: de-mineralization inhibition (when present in liquid phase), re-mineralization enhancement as well as inhibition of bacteria found in the dental plaque.2
Fluoride exerts its main anti-caries effect on the enamel of teeth; it can also exert an antimicrobial effect, which, although subtle, can be extremely important. Although fluoride cannot directly alter micro-flora composition it can act by preserving plaque's microbial homeostasis, exerting a stabilizing effect upon sugar concentration and pH variation during oscillatory conditions.1
One of the mechanisms through which oral environment homeostasis can be interrupted is through exposition of plaque to a low pH, which can be caused by the frequent intake of fermentable carbohydrates. A suitable prevention mode could be related to a decrease of intake of foods with high contents of fermentable carbohydrates. Another prevention mode would be the use of sugar substitutes, since they cannot be metabolized by dental plaque's micro-organisms.1
Sugar substitutes possess the ability of reducing at least one of the four essential etiological factors (diet, micro-flora, susceptibility and time) for dental caries,1,2 this is the presence of fermentable carbohydrates in the diet in order to break interaction of susceptible teeth with cariogenic bacteria of the plaque and sugar, all of which are causing agents for the disorder.1,3-5
Xylitol is one of the most suitable and promising sugar substitutes tested for caries prevention purposes. It is as sweet as saccharose (common sugar) and cannot be metabolized by most of oral bacteriae.3,5-9
In the process of caries prevention, dental brushing with a fluoride toothpaste has become a public measure of oral health. Since fluoride concentration found in toothpastes does not provide comprehensive protection for all subjects, and must observe certain limits due to legal regulations, there is doubt upon whether to effectively use additional products with fluoride content as well as incorporation of other ingredients.10
Xylitol or pentinol is a natural, 5 carbon sugary alchohol which has proven to be an effective agent in caries prevention in animals and humans. It is naturally found in some fruits and vegetables.1,3-5 Many countries have approved its consumption in the daily diet. Presently, it is incorporated as sweetener into various products such as sweets, chewing gums, confectioneries, as well as into oral hygiene products, cosmetics and medical drugs.3,6-8
The mechanism and re-mineralizing effect of xylitol are triggered into action whenever chewing gum of food containing materials which stimulate salivation cannot be fermented. In those cases, plaque and dental surface found underneath plaque result exposed to an environment exhibiting a pH very similar to the salivary pH, this favors the process of tooth remineralization. When these events occur repeatedly, after de-mineralization episodes, it is probable that potential clinically important re-mineralization episodes will occur.6
Therefore, caries clinical studies have reported what was called reversions of early caries lesions (white spots) with unusually high frequencies amongst users of xylitol containing chewing gum.6
Former research on xylitol mode of action revealed that fermentation was nil due to most microotganisms of dental plaque. The following was equally observed: absence of significant degradation of dental plaque to acid terminal products, stimulation of salivary flow, increase in buffering capacity inhibition of cariogenic bacteria and plaque accumulation, re-mineralization of de-calcified areas as well as inhibition of healthy enamel de-mineralization.3,6,8,11,12
Xylitol has recently been incorporated into fluoride tooth pastes and mouthwashes. In vitro studies suggest the fact that the aforementioned ingredients exert additional inhibiting effect upon growth and/or production of cariogenic microorganism acids.5,9
Many studies conducted with pure bacterial cultures, dental plaque suspension and in situ pH measurements have established the fact that xylitol meets all criteria to be used as dental caries preventive agent.3,12,13
MATERIALS AND METHODS
The sample was composed of 40 caries-free primary teeth, randomly selected and about to exfoliate. They were divided into five groups of eight teeth each. Once the teeth were extracted, infection control procedures were undertaken so as to guarantee bio-security in the handling of biological specimens. Therefore, after teeth were extracted, they were immersed in a 6% NaOCl solution in order to neutralize any bacterial or biological component found in the sample. Samples were then rinsed with abundant de-ionized water until total removal of any organic remain on the surface. Once cleansed, specimens were stored in de-ionized water. Crowns were varnished leaving a free 3 x 3 mm surface. The surface was later treated with a 35% phosphoric acid gel during 20 seconds. Teeth were then rinsed with tap water; a control group was set aside and left in de-ionized water, the other four groups were immersed in a rinse of 0.5 g sodium fluoride and 1% xylitol (Fluoxytil®).
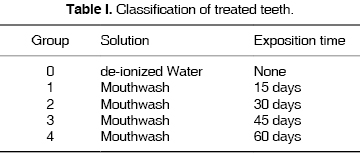
Groups were structured so as to have one control group and four experimental groups (according to exposition and observation times)(0 to 60 days), as shown in table I. The control sample as well as samples immersed in xylitol and fluoride rinse were stored in sealed glass jars, and were taken to a Hanau oven to achieve a constant 37 oC temperature, with absolute humidity. The rinse was changed on alternate days.
Once the oral rinse exposition term was completed, specimens were mounted on plastic supports and fixated with self-curing acrylic. At a later point they were placed on a diamond disk trimmer so as to undertake 150-250 µm longitudinal cuts (Figure 1). These cuts were observed, and re-mineralization was assessed in function of refraction exhibited by the cuts after application of Thoulet 1.47 IR solution.

Samples were observed under polarized light in a photomicroscope (Axiophot, Zeiss, Germany) with 5X and 10 X work objectives (Figure 2) so as to determine existing bi-refringence. The following criteria were applied (Table II).


In order to determine surface re-mineralization, photographic records were taken of all specimens at different experimental periods (15, 30, 45 and 60 days) respectively, the records were compared to the control group.
RESULTS
Groups were observed according to their corresponding period. In all sessions, photographic records were taken and a previously determined measurement scale was applied. Data were gathered into statistical collection cards and Anova variance analysis was applied. This procedure was conducted in order to establish the existence of statistically significant differences among groups (Figure 3).

Control surface was treated with 35% phosphoric acid. These surfaces were not rinsed (Figure 4).

In the 15-day group, superficial changes were observed. A 0.444 mean was established (± 0.27 DE). In that group, the surface treated with phosphoric acid did not show significant changes when compared with the control group. Control group also underwent superficial treatment but was not exposed to rinsing (Figure 5).

In the 30-day group, changes observed under polarized light were more evident than in the 15-day group. Nevertheless, these changes did not reach beyond enamel surface. In this group the mean of changes observed in enamel was 0.778 (± 0.441), which was almost double the change observed in the former group (Figure 6).

In the 45-day group a 1.44 mean (± 0.527 DE) was determined. This meant that in all cases there was at least one change in enamel surface, and this change was not only limited to the surface, a sub-surface characteristic modification was observed as well (Figure 7).

The 60-day group exhibited a 1.47 mean (± 0.483). This showed the fact that changes were found underneath the enamel surface (Figure 8).

Upon application of statistical analysis, it was determined that there was statistically significant difference among groups when compared with control group (H = 21.992, p ≤ 0.001). Differences were found even among different groups. In the same manner, an attempt was made to determine which were the associations when comparisons among groups were established. For this reason, Dunnet multiple comparison method was applied. With it it was found that there were statistically significant differences (p < 0.05). This could be interpreted as changes in enamel caused by the effect of the rinse in all studied periods.
DISCUSSION
Re-mineralization and de-mineralization processes which take place in dental tissues are dynamic mechanisms which are carried out during the time in which the tooth remains in the mouth.3,4 For example, an unbalanced environment generated by pH and saliva as endogenous factors, together with diet as exogenous factor, allow bacteria to not only be the causing agents for a de-mineralization phenomenon, but also to contribute to it. On a contrary basis, a balanced oral environment favors the re-mineralization process.3,4,6
Fluoride use is an undisputed viable and cost-effective method. This is probably still the situation in countries where there are not nationally-minded preventive systems, or those countries with relatively high caries incidence.2
Apparently, when fluoride oral rinse is used as an adjuvant of unsupervised oral hygiene, with a fluoride toothpaste, caries inhibition is approximately 10 to 20%. In this context, it is important to observe that effectiveness of any caries prevention measure not only depends on the isolated measure, it also depends on other factors such a caries risk, awareness of dental health, and presence of organized dental health care. Therefore, one fluoride rinse program could result effective in one type of population, while remaining ineffective in others.13
Reports on xylitol used in toothpaste upon saliva and caries incidence have been contradictory. Effect of xylitol in mouthwash upon saliva and plaque have been scarcely studied.5
In scientific literature, there are reports which suggest a re-mineralization effect based upon different compounds. One example would be glass-ionomer cements, which can be directly or indirectly applied on enamel surfaces.3,4,6 Purportedly they exert a re-mineralizing effect due to the interaction and concentration of the fluoride ion from the fluoride-aluminum-silicate which forms part of the matrix of these cements.6 The last decade has seen the development of alternative treatments to reduce carious lesions incidence, as well as resources to obtain re-mineralization areas through procedures which although simple, are very valuable for the prevention of this disease.3,4,6 In fact, enamel surface re-mineralization function has been attributed to xylitol based compounds.3,4-6,9,12
As was the case with Amaechi,6 in the present research, an experimental model was applied with the aim of assessing the re-mineralizing ability exhibited by the aforementioned compound, with the difference that our study employed primary human teeth and not bovine teeth. Results of the present study concur with other results reported in literature inasmuch as they suggest lesser mineral loss as well as reduced cariogenic potential due to its re-mineralizing activity.1,3,4-6,9
Wennerholm & al13 reported a study where, after having used chewing gum with xylitol for a 21-25 days period, they discovered decrease of salivary pH. That study was conducted in vivo in adult patients.
In a similar manner, Gertsen & al5 could not prove the effect of xylitol and fluoride mouthwashes ( by themselves or combined) upon microflora or salivary secretion indexes, on the accumulation of dental plaque, on the development of gingivitis or upon plaque's acidogenic potential . A possible explanation for the finding of contradictory results in several research projects could be the fact that the xylitol content in the vehicle varies greatly, another fact to consider could be the diversity of presentations and differences in the frequency and duration of use.5,9
Besides the already mentioned variations, in the different studies there are other variants tailored to intended objectives. Very few studies purported the same materials and methods. For this reason, the present study could hardly totally coincide with other consulted research. In order to better assess the product effectiveness, it would probably be necessary to allow teeth to remain longer time in contact with the fluoride and xylitol solution.
The determination of changes elicited by polarized light upon mineral components is a technique used to assess re-mineralizing effects. Results showed the aforementioned re-mineralizing effect on at least the enamel surface in the 45 day period. Even though mechanisms involved in homeostasis of enamel re-mineralization processes are complex, the role played by xylitol when assessed under polarized light cannot be discarded, at least that was the case in the present study.
To be more knowledgeable on these variables, evaluation time must be longer. It is also necessary for experimental conditions to consider variables such as presence of certain electrolytes, and, whenever possible, simulation of dental vitality environments. Moreover, application of techniques for the characterization of enamel surface must be considered, so as to allow changes in the composition. It would then be reasonable to hope achievement of these phenomena through use of chemical and optical characterization instruments so as to determine changes in the enamel structure.
CONCLUSIONS
In the present study, mouthwash with sodium fluoride and xylitol exerted a slight re-mineralizing effect upon surface and sub-surface of enamel of primary teeth.
REFERENCES
1. Trahan L. Xylitol: a review of its action on mutans atreptococci and dental plaque- its clinical significance. International Dental Journal . 1995; 45: 77-92. [ Links ]
2. Mäkinen KK, Isotupa PK, Kivilompolo T, Makinen LP, Tiovanen J, Soderling E. Comparison of erythritol and xylitol saliva stimulants in the control of dental plaque and mutans streptococci. Caries Reserch . 2001; 35: 129-135. [ Links ]
3. Amaechi BT, Higham SM, Edgar WM. The influence of xylitol and fluoride on dental erosion in vitro . Archives of Oral Biology . 1998; 43: 157-161. [ Links ]
4. Tanzer MJ. Xylitol chewing gum and dental caries. International Dental Journal . 1995; 45: 65-76. [ Links ]
5. Hayes C. The effect of non-cariogenic sweeteners on the prevention of dental caries: a review of the prevention of the evidence. J Dent Educ . 2001; 65: 1106-1109. [ Links ]
6. Söderling E, Isokangas P, Pienihäkkinen K, Tenovuo J, Alanen P. Influence of maternal xylitol consumption on mother-child transmission of mutans streptococci: 6- year follow-up. Caries Research . 2001; 35: 173-177. [ Links ]
7. Roberts CM, Riedy AC, Coldwell ES, Nagahama S, Judge K, Lam M. How xylitol-containing products affect cariogenic bacteria. American Dental Association . 2002; 133: 435-441. [ Links ]
8. Wennerholm K, Arends J, Rubrn J, Emilson GC, Dijkman GA. Effect of xylitol and sorbitol in chewing-gums on mutans streptococci, plaque pH and mineral loss of enamel. Caries Res . 1994; 28: 48-54. [ Links ]
9. Lopes ANC, Valsecki JA, De Sousa SSL, Carife BG. Efeito de solucoes fluoretada contendo xilitol e sorbitol no número de streptococos do grupo mutans na saliva de seres humanos. Rev Panam Salud Publica . 2001; 9 (1): 30-34. [ Links ]
10. Giertsen E, Emberland H, Scheiet. Effect of mouth rinses with xilitol and fluoride on dental plaque and saliva. Caries Res . 1999; 33: 23-31. [ Links ]
11. Jannesson L, Renvert S, Birkhed D. Effect of xylitol in an enzyme-containing dentifrice without sodium lauryl sulfate on mutans streptococci in vivo . Acta Odontol Scand . 1997; 55: 212-216. [ Links ]
12. Campus G, Lallai RM, Carboni R. Fluoride concentration in saliva after use of oral hygiene products. Caries Research . 2003; 37: 66-70. [ Links ]
13. Zimmer S. Caries-preventive effects of fluoride products when used in conjunction with fluoride dentifrice. Caries Research . 2001; 35: 18-21. [ Links ]
Note This article can be read in its full version in the following page http://www.medigraphic.com/facultadodontologiaunam Mailing address:
Mailing address:
Emilia Valenzuela Espinoza
E-mail: emy_valenzuela@hotmail.com











 text in
text in 


