Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista odontológica mexicana
versión impresa ISSN 1870-199X
Rev. Odont. Mex vol.17 no.3 Ciudad de México jul./sep. 2013
Original research
Leukocyte death incited by propolis toxicity
VC Tinoco Cabriales,* JA Quesada Castillo,* MA Maldonado Ramírez,* R Oliver Parra,* BA Luna Gojon§
* Research Professors. School of Dentistry of the UAT (Universidad Autónoma de Tamaulipas)
§ Pedodontics Graduate Student. School of Dentistry of the UAT (Universidad Autónoma de Tamaulipas)
ABSTRACT
Objective: The aim of the present study was to assess the extent of propolis' in vitro cytotoxicity on polymorphonuclear leukocytes.
Materials and methods: The present study was an in vitro, controlled, experimental endeavor. Statistical procedure entailed variance analysis of repeated measures as well as Scheffe's post-hoc. A p < 0.05 statistical significance was established. To obtain leukocytes, 10 mL of peripheral venous blood was harvested from six randomly selected, healthy, 20-30 year old subjects, of both genders.
Results: Scheffe's analysis with 95% reliability for comparison between control and experimental groups. Significant with p 0.0001 for propolis 1 control 1 and propolis 2 control 2. For propolis 1 propolis 2 p 0.5002/control 1 control 2 p 0.9621.
Conclusions: In the present experiment propolis at a 1:4 dilution applied for 1-2 hours to polymorphonuclear leukocytes caused 70% cellular death. This resulted in statistical significance.
Key words: Cytotoxicity, propolis, neutrophil polymorphonuclear leukocytes.
INTRODUCTION
Recently, in the area of natural products research, a material called propolis stands out; scientific literature reports this materials is analgesic, fungicide, anti-inflammatory, healing as well as anti-cariogenic.1
Bees produce this resin through a process of mixing substances gathered from budding plants, flower buds and resinous exudates. They thus produce a material fit to close gaps, embalm dead insects within the beehive, as well as protect them from micro-organism and insect invasion.2 Inca tribes used it to cure febrile infections. In XIII and XIV century Europe, it was used to treat sores. Its therapeutic action was attributed to the several phenolic compounds which conform it, among which the main are flavonoids, as well as some phenolic acids, esters, aldehydes, alcohols and ketones.3,4,5
Propolis physical and chemical composition is as follows:
• Resins and aromatic balsams 50-80%.
• Essential oils and other volatile substances (4.5 to 15%).
• Waxes (12-15%).
• Pollen (5-11%).
• Flavones, flavonoids, flavanones, dihydroflavones.
• Benzyl alcohol, benzalcenid, benzoic acid.
• Cinnamic alcohol derivatives, coumarins, phenolic triglycerides.
• Other aromatic elements, monoterpenes, hexaterpenes, triterpenes.
• Polyunsaturated fatty acids and linoleic acid.
• Vitamins A, B1, B2, B6, C, E, Nicotinic acid, pantothenic acid.
• Copper, manganese, magnesium, nickel, silver, silica, vanadium zinc.
When a subject is born, it is exposed to numberless microorganisms heretofore unknown to him. They can become part of his normal flora population, or they can cause illness which will elicit as a consequence a certain type of immune response, which can be non-specific, where neutrophil polymorphonuclear leukocytes intervene (NPML), or a specific response where there is interacting of T and B lymphocytes.6,7
NPML are the main phagocytic cells found in the peripheral blood, they conform 50-70% of total white blood cells7. They are considered the second defense line of the human body, the first line of defense being skin and mucosa.6 Average life is 8-20 hrs in circulation, which increases when entering infected or inflamed tissues.8
NPML transit from the capillaries until reaching the lesion site experiences several phases: 1 margination NPML contact with endothelial walls. 2. Endothelial adherence through selectins and integrins. 3. Diapedesis. trans-endothelial migration. For this to happen, chemotaxis is necessary, that is to say NPML must be attracted towards the infection focus through different molecules such as IL-8 (interleukin 8), complement C5a factor of the complement, LTB4 (leukotriene B4) among others. Phagocytosis and cellular death. Destruction of the micro-organism within the neutrophil takes place through two mechanisms; one oxygen dependent and one oxygen-independent.9
The neutrophil dies, once it has fulfilled its function, following an apoptosis procedure (programmed cellular death). This entails certain characteristic alterations such as the increase in phosphatidylserine surface markers' expression. This helps in the elimination undertaken by macrophages, avoiding thus possible cytotoxic content release into the extra-cellular medium. This represents a benefit of the apopoptic neutrophil inasmuch as it reduces inappropriate inflammatory response. The model is based upon the strategy of providing benefits to excessive responses in a microbial infection.10 In our NPML research we have developed a model to isolate and purify NPML from peripheral blood so as to later confront them to different substances, propolis in this case, to measure its ability to exert cytotoxic effect.
MATERIALS AND METHODS
This study was an in vitro, controlled, experimental study with the following statistical procedure: repeated measurement variance analysis and Scheffe's post-hoc p < 0.05 statistical significance. Harvested blood was mixed with heparin (Inhepar) in the following proportion: 5,000 U per blood ml, to avoid coagulation and leucocyte entrapment in 16 x 150 test tubes with 3 mL of 3% Dextran (Sigma co). From the previous tube, blood was passed onto another tube where 3% Dextran was previously incorporated. This procedure must be carefully performed with sterile Pasteur pipette, attempting to achieve blood sliding on the tube's walls so that red blood cells do not lyse. Incubation was conducted at 37°C for one hour, in an attempt to replicate human body conditions. Plasma rich in leukocytes was gathered in 13 x 100 tubes. Upon withdrawing plasma from the incubator, two phases could be observed: a leukocyte-rich upper or supernatant phase and a lower or sediment phase mainly composed of red blood cells and platelets. Blood was centrifuged at 180g (1,200 rpm) during three minutes (Ficher Scientific centrifuge). Cell free supernantant material was discarded, and leukocytes remained agglutinated at the bottom of the tube. In order to eliminate Dextran excess, three rinses were performed with RPMI 1640 commercial culture medium. Cells were then gently separated to then undertake counting. Obtained cell population was adjusted to a 12 x 108 cell concentration per mL. 5% acetic acid was incorporated to achieve better leukocyte counting; the following were used as adjuvants: a Neubauer camera, Leica and Van Guard light microscopes as well as a manual counter
Experimental design.
Our in vitro system counted with the following reactive agents:
1. Problem sample tube containing propolis, cells RPM!-1640 medium tested at one and two hours. Propolis was at 1:4 solution rate (minimum inhibitory concentration obtained in former experiments performed by Lara, Tinoco et al).11
2. Viability control conducted with cells and RPMI-1640 medium as control group. A sample of each group at one and two hours was left in the incubator at 37°C. Results were observed by counting dead and live cells using 5% Trypan blue dye. Blue-dyed cells were considered dead or non-viable, due to the entrance of the dye through membrane and cellular walls. Live, refringent cells showed no coloring and no cellular damage. The tubes then received 0.5 mL propolis at 1.4 concentration, then 0.5 mL cells, then medium. After incubating for one and two hours images were observed. Images exhibited provoked agglutination as well as blue-tinted polymorphonuclear leukocytes which were evidence of cellular death.
3. Purity in NPML obtention. The separation of neutrophil polymorphonuclear leukocytes was undertaken with 95 to 100% purity as demonstrated by Wright Stain procedure, under microscope at 40x objective.
RESULTS
In figure 1 we can observe a leukocyte obtained through Dextran purification technique, dyed with Wright stain. Viability range with this method was fairly high: it reached 95-100% purity.
In figure 2, here we can observe live leukocytes not affected by propolis since they appeared hyaline and did not incorporate Trypan blue stain. Dead NPML would result dyed in blue.
In figure 3, strong agglutination elicited by propolis can be observed, in the figure 4. Dead leukocytes can be observed when incorporating 5% Trypan blue stain.
Quantified results previously shown statistically express the following based on six performed repetitions:
In table I we find results of the six experiments, where the number of live and dead cells is counted after one hour and two hour periods. Control was basal count of 12,000,000 Neutrophil polymorphonuclear leukocytes. (NPML). Subsequently, in all experiments, the number of dead and live NPML were counted after one and two hour periods. Results of the present experiment showed, when viability was assessed in the control group, a population approximately three times greater than in the group where propolis was present.
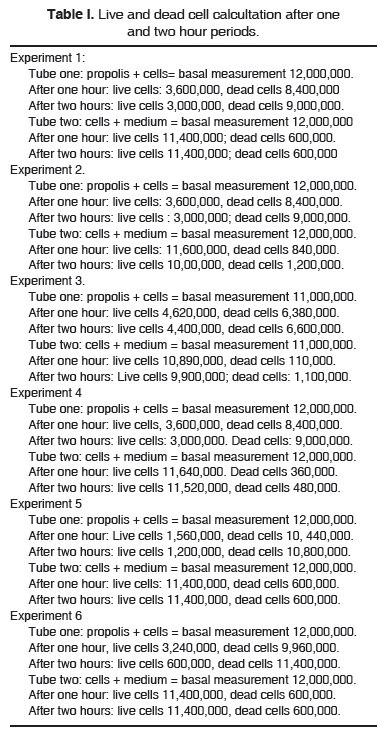
Initial basal measurement, in all experiments, was adjusted to 12,000,000 NMPL. Population was thus standardized to avoid fluctuations which might have affected results.
Statistical analysis showed significant statistical difference between propolis 1 (measured at 1 hour of incubation time) and control 1 with p < 0.0001 value. The same could be said for propolis 2 (measured at two hours of incubation time ) when compared to control two with a p < 0.0001 value.
Analysis showed the following (Table II):
DISCUSSION
The antimicrobial ability of propolis has been widely researched and demonstrated by Bretz et al, Koo et al, Hegazi AG, El Hardy FK as well as Drago M et al.2,4,11-13 Propolis's toxicity has not been extensively researched and few studies on live cells have been recorded. This is one of the reasons behind the use of polymorphonuclear leukocytes in the present experiment since they are easily recoverable cells of peripheral blood as well as second line of defense of the human body; they are equally involved in anti-bacterial and anti-infectious processes, which are essential characteristics mentioned here for propolis as well.
Leukocyte separation was based on the method used by Arce Mendoza, Tinoco Carbiales (1984) when they measured leukocyte chemotaxis on tetracycline effect.14,15
Results reflect the fact that cell harvesting purity was adequate, since cells dyed following Wright's method appeared with a purplish hue with well-circumscribed nuclei (Figure 1). Our viability control was high (95-100%). In it, leukocytes appeared refractile, therefore alive.
In the test tube where propolis was incorporated into the cells a patent agglutination could be observed. The present study thus proposes this might be one of the anti-bacterial effect mechanisms subject of study by several authors such as Koo, Parl et al.4,16
Cell populations used in the present study (basal measurement) were constant (standardized), that is, 12,000,000 leukocytes as initial cell population. Upon counting, control group exhibited three times the viability than that observed when propolis was used. This result was repetitive in the different quantified samples. There was no apparent significant difference with results obtained at one or two hours. This would substantiate the theory that propolis is indeed acting with constant toxicity on the cells. This situation coincides with reports of Scheller et al: working with mice they applied ethanol and propolis extract and found pathological changes in the liver which were transitory and reversible in a period of two to four weeks after intra-venous administration.
Some authors, like Ramirez et al17 support the theory that high doses of propolis orally administered to animals (10 to 15 mg per kg of bodyweight) do not elicit toxic effects or pathological disorders, even in the long term. This might show certain relationship to a dosage-effect response.
The results obtained in the present study were not significant when considering the time variable. The differences in the control group after one hour showed p < 0.0001 statistically significant differences. This was also the case for the two hour group and its control p < 0.0001. In the light of results reported by Magro and Fhilo and Carballo18 this would result paradoxical since they reported the fact that propolis elicited accelerated epithelial repair after rat extraction.
Seheller et al, in a study conducted on dogs, found stimulation in the regenerative process. Stojko and Seheller, in a study conducted on dogs reported osteogenesis acceleration as well as bone tissue regeneration with pronounced anti-bacterial effect. It should be noted that the aforementioned authors were not looking for measurements of cellular death. We could surmise they were using different ways for propolis preparation or extraction, which could alter results. Propolis, as an alternative product does not have precise standardization.
Finally, it must be pointed out that experiments conducted by us were in vitro. When interacting in vivo, elements implicated in the present experiment could sustain a buffer effect elicited by the organism, since, when propolis is diluted in our human system its toxic effect on NPML could be reduced to a minimum. Nevertheless, this would not be the case for its bactericidal or anti-inflammatory effect.
REFERENCES
1. Young K, Matías S, Lima C. Comparação das características fisico químicas das própolis producidas na região sub-tropical da América do Sul; evidencia firoquímica de sua origem botánica. Mesagem Doce. 2001; 61. [ Links ]
2. Yong Kun Park, Ikegaki M, de Alencar SM. Clasificação das própolis brasileira a partir de suas características físico-químicas e propriedades biológicas. Mesagem Doce 2000; 58. [ Links ]
3. Bankova V, Christov R, Hegazi A.G, ABD El Hady, Popov S. Chemical composition of propolis from popular budws. International Simposium on Aphiterapy Cairo 1997; 8-9. [ Links ]
4. Dos Santos A, Mendes da Silva JF, Kiltzke R, Nobrega J, de Aquino Neto FR. Identificação do esteres graxos de triterpenoi des pentaciclicos em própolis. Mesagem Doce 54. [ Links ]
5. Almas K, Mahmoud A, Dahlan A. A comparative study of propolis and saline applications on human dentin. A SEM study. Indian J Dent Res. 2001; 12 (1): 217. [ Links ]
6. Anaya JM y cols. Inmunología (de memoria). Cap. 4, p. 39, 15a Ed. Corporación para investigaciones biológicas 2009. México, D.F. [ Links ]
7. Guyton A. Fisiología y fisiopatología básicas. 4a ed. Ed. Interamericana 1980. Barcelona, España. Pte. II Células de la sangre, inmunidad y coagulación. [ Links ]
8. Edwards SW. Biochemistry and phisiology of the neutrophil. Cambridgen University Press 1994. New York. [ Links ]
9. Brobeck JR, Best R. Bases fisiológicas de la práctica médica. 10a ed. Ed. Panamericana, República de Argentina. 2006. [ Links ]
10. Jenkins N. Fisiología y bioquímica bucal. 1a ed. Ed. Limusa México 1983: 179-212. [ Links ]
11. Lara M y cols. Estudio in vitro de la actividad antibacteriana del propóleo contra microorganismos de la placa dentobacteriana supragingival. Tesis 2001 Fac de Odontología UAT. [ Links ]
12. Katzung G. Farmacología básica y clínica. Ed. Manual Moderno. México. 1984. [ Links ]
13. Isla M, Nieva, Moreno M, Samprieto A, Vattuone M. Antioxidant activity of Argentine propolis extract. J Ethopharmacoloc. 2001; 76 (2): 165-170. [ Links ]
14. Arce MA y cols. Efecto de las tetraciclinas sobre la quimiotaxis leucocitaria, estudio doble ciego en pacientes con acné vulgaris. Tesis 1983. Monterrey N.L. [ Links ]
15. Rivera y cols. Evaluación de la fagocitosis y digestión intracelular por leucocitos polimorfonucleares de individuos sanos para dos especies de estafilococos coagulasa negativo. Tesis para obtener grado de maestría. UANL Fac de Medicina. 1990 Monterrey N.L. [ Links ]
16. Koo H, Rosalen P et al. Effects of apis mellifera propolis from two brazilian regions on caries development in desalivated rats. Caries Res. 1999; 33 (5): 393-400. [ Links ]
17. Ramírez ME, Villalobos DE, Villafuerte GA, Andrade FF. Propóleo: ¿Una alternativa en la terapéutica médica y odontológica? Primera parte Med Oral. 2001; 2: 91-99. [ Links ]
18. Magro O, Carvalho A. Application of propolis to dental sockets and skin wound. J Nihon Univ Sch Dental. 1999; 36 (2): 4-10. [ Links ]
RECOMMENDED LITERATURE — Samson y Wright. Fisiología aplicada. La sangre, los glóbulosrojos en la sangre. 5a ed. Sección II, Ed. Marin S.A.; Barcelona España. 1966. — Asis M. Apiterapia para todos, cómo usar los siete productosde la colmena para curar. Editorial Científico Técnica. 1993. La Habana Cuba. — Makashvili ZA. From the history of propolis, in remarkable hive products: propolis, scientific data and suggestions concerning its composition, properties and possible use in therapeutics. Apimondia standing commission on beekeeping technology and equipment. Apimondia Archivosde la Federación Internacional de Asociaciones de Apicultura1978. Bucharest. — Hajdaragi I. The effects of propolis on the reparative processes of the pulp and histological analysis of the pulp 28 days after artificial exposure and covering with propolis. Stomatol Vjesn. 1983; 12 (3-4): 111-114. — Quintana J, Alonso O, Díaz M, Lópes M. Empleo de la tintíra de propóleo al 5% en la cura de heridas sépticas faciales. RevCubana Estomatol. 1997; 34 (1): 25-27. — Bretz WA, Chiego DJ et al. Preliminary report of the effects of propolis on wound healing in the dental pulp. Z Naturforsch C. 1998; 53 (11-12): 1045-1048. — Scheller S, Luciak J, Koziol M et al. Biological properties and clinical application of propolis. VIII, experimental observation on influence of ethanol extracts of propolis on regeneration of bone tissue. Arzneim-Forsch Ed Cantor. Drug Res. 1978; 28 (II)9: 1594-1595. — Gafar M, Dimitriu H, Smichise E. Preparados farmacéuticos con extracto de propóleos, empleados en el tratamiento de las parodontopatías marginales crónicas. SimposiumInternacional de Apiterapia, Apimondia, Bucarest. 1976; 20: 189-192. — Khayal M, El Ghazaly MA. Mechanisms involved in the antiinflammatory effect of propolis extract. Drugs Exptt Clin Res. 1993; XIX (5): 197-203. — Drago L, Mombelli B, De Vecchi E, Fascina M, Tocalli L, Gismondo M R. In vitro antimicrobial activity of propolis dry extract. Chemother. 2000; 12 (5): 390-395. — Vasilev V, Monova KSE, Todorov V. Tratamiento con propóleos en la moniliasis e intertigo de los lactante. Propóleos Investigaciones científicas y opiniones acerca de su composición y utilización con fines terapéuticos. Apimondia Archivos dela Federación Internacional de Asociaciones de Apicultura.Bucharest. 1975: 274-275. Note This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam Mailing address:
Mailing address:
Jorge A Quesada Castillo
E-mail: jquesada@uat.edu.mx











 texto en
texto en 


