Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista odontológica mexicana
versión impresa ISSN 1870-199X
Rev. Odont. Mex vol.17 no.3 Ciudad de México jul./sep. 2013
Original research
Osteocalcin expression in periodontal ligament when inducing orthodontic forces
Maritere Villarreal Brito,* Marco Antonio Álvarez Pérez,§ Francisco Javier Marichi RodríguezII
* Orthodontics Program student, Graduate and Research School, National School of Dentistry.
§ Professor and Researcher, Molecular and Cellular Biology Laboratory, Graduate and Research School, National School of Dentistry.
II Orthodontics Specialty Professor, Graduate and Research School, National School of Dentistry. National University of Mexico (UNAM)
ABSTRACT
Osteocalcin is a non-collagenous protein located in alveolar bone, root cementum and subpopulations of periodontal ligament cells. This protein plays an important role in the biomineralization process and in the extra-cellular matrix, regulating maturation of hydroxyapatite and osteoclast recruitment which participate in bone remodeling. Periodontal tissue new formation and remodeling is a vital part of the process during orthodontic movements. These movements, when force is exerted, cause tension in the cells, provoking adaptation which results in molecular and cellular responses which, in turn, can affect the extracellular matrix. Due to the aforementioned facts, the aim of the present research was to determine osteocalcin expression associated to periodontal remodeling when orthodontic forces are applied. Roth 0.022 " fixed brackets with a NiTi 0.016" archwire were applied to first upper and lower bicuspids. This was applied to all teeth of both arches except to left lower and upper bicuspids. Bicuspids without brackets (t = 0) as well as with brackets to elicit orthodontic movements during 1, 3, 5, 7 and 9 days were extracted to assess osteocalcin expression in the extra-cellular matrix of the periodontal ligament. The RT-PCR technique was followed to determine temporal and spatial expression of osteocalcin messengers. Osteocalcin expression in the experimental group was present in all test days, suggesting thus the fact that orthodontic movements elicit changes that are susceptible in osteocalcin protein messenger concentrations.
Key words: Osteocalcin, periodontal ligament, orthodontic forces.
INTRODUCTION
Osteocalcin, or BGP (Bone Gla Protein) is the non-collagenous protein most abundant in the periodontium. It constitutes up to 3% of total bone protein. It contains 49 amino-acids with three gamma-carboxyglutamic acid residues, which are associated to its calcium-bonding properties. BGP is produced by osteoblasts, cementoblasts and periodontal ligament fibroblast subpopulations, totally differentiated with an 6kDa approximate mass. It is secreted at the mineralization site.1-3
Immuno-histochemistry studies have located BGP in alveolar bone, dentin and root cement. Its role in the biomineralization process has been partially determined. It is known that osteocalcin production is stimulated by vitamin K, since, in animal models treated with warfarin (vitamin K antagonist), inhibition of carboxylation of glutamic acid residues was observed, and to the type 2 (FGF-") fibroblastic growth factor , since gene FGF-«knock out» mice presented bone hypermineralization. This suggested the fact that BGP plays a role in the biomineralization process as well as in the extracellular matrix by regulating hydroxyapatite cristal maturation and osteoclasts recruiting participating in bone remodelling.4-7
During orthodontic treatment, teeth respond to exerted mechanical forces by remodeling the extracellular matrix of the periodontium. When forces are exerted upon a tooth there are two main points which might influence (elicit) a response: a pressure side where resorption cycles occur, and a tension side, where extracellular matrix formation predominates. During the processes of resorption and periodontium extracellular matrix formation, under the influence of orthodontic movements, there is expression of a great number of molecules (growth factors, collagen molecules, glycoproteins, hormones, proteoglycans etc.) to regulate the functions of cellular adhesion, motility growth and differentiation. These functions are firmly regulated by the extracellular matrix and play a key role in orthodontic movements.8
Research conducted on orthodontic movements have reported that local application of osteocalcin produced acceleration of dental movement in rats during initial treatment phases. This acceleration was mainly due to an increase of osteoclast recruitment. This had led to suppose that osteocalcin application at initial stages can be sufficient to accelerate dental movement, since it could act as a molecule promoting chemo-attraction for osteoclasts precursor cells, participating thus in the remodeling of the matrix as well as favoring a suitable micro-environment for biomineralization.8,9
For all the aforementioned reasons, the aim of the present study was to determine the expression of the gene which codifies osteocalcin found in the periodontal ligament of teeth subjected to orthodontic forces during 1, 3, 5, 7 and 9 treatment days.
METHODS
PATIENT SELECTION AND FIXED APPLIANCES PLACEMENT
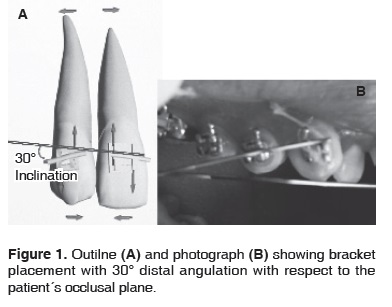
To conduct the present study, 50 patients were selected. Patients were healthy, of both genders, over 18 years of age, and attended the Orthodontics Clinic at the Graduate and Research Division of the National School of Dentistry, UNAM. Base treatment contemplated extraction of first upper and lower premolars. Patients were informed of the study and granted consent. The 50 patients were randomly divided into 10 patient groups to be allotted to each experimental period (1, 3, 5 7 and 9 days). Roth 0.022" fixed brackets were placed with NiTi 0.016" gauge. Sentalloy archwire. Brackets were placed on all teeth of both arches, except for left upper bicuspids which acted as control group (t = 0). The study involved a split mouth design where right upper bicuspids were considered as the group subjected to orthodontic pressure (experimental group). To these bicuspids, a bracket was applied which exhibited a 30° distal angulation with respect to the patient's occlusal plane (Figure 1). This bracket was ligated to the archwire with an elastomeric module (GAC brand), and the tooth was subjected to gauged, constant orthodontic pressure during spans of 1, 3, 5, 7 and 9 days. Upon completion of each period in which orthodontic forces were applied, upper and lower bicuspids were extracted.
DENTAL EXTRACTION
Patient premolar extraction was performed at the Periodontics Clinic of this same institution at intervals of 1, 3, 5, 7 and 9 days after bracket placement. Extractions were achieved with the help of 1.8 ml lidocaine and epinephrine anesthetic (36 mg/0.025 mg, Uniseal). Teeth were luxated with straight elevator Number 301 m and were manipulated with number 150 forceps. Extracted bicuspids were placed in liquid nitrogen for conservation and later use for RNA isolation.
RNA ISOLATION
Bicuspids subjected to orthodontic movements (t = 1, 3, 5, 7 and 9 days) and not subjected to orthodontic movements (t = 0) were extracted in order to analyze osteocalcin gene expression present in the periodontium extra-cellular matrix. After bicuspid extraction, periodontal tissue bonded to bicuspid root's medial and apical zone was removed by scraping with a scalpel blade. Tissues were homogenized in 1 mL TRIZOL (Gibco BRL) so as to conduct complete RNA extraction following manufacturer's indications. Total RNA was incubated with DNAase free of RNAase (Boehringer, Mannheim Biomedicals, IN) with the aim of guaranteeing absence of genomic DNA contamination. RNA was quantified with the help of 260nm optic densitometry and was later used for the RT-PCR technique.
RT-PCR
RT-PCR technique was executed in order to determine spatial and temporary expression of osteocalcin messenger isolated from periodontal extracellular matrix subjected to orthodontic movements at 1, 3, 5, 7 and 9 days. Super-Script One-step RT-PCR kit was used (Invitrogen, Carslbad CA) for transcription of the complementary chain from 1 µg of total RNA in each sample, following manufacturer's specifications. To generate each cDNA template, RT was achieved, osteocalcin specific oligo in sense - direction 5' GATCCATGAGAATGAGAAGCG 3'and direction-anti-sense 5'CTATGTTAGCACCTTATCCCC 3' at 45°C during 30minutes, and a 94°C cycle for two minutes. cDNA was amplified under the following PCR program: 94°C during one minute ( de-naturalization), 55°C alignment temperature for the osteocalcin oligos during one minute, and extension to 72°C for one minute. This was performed until completing all 35 cycles. PCR products were separated with electrophoresis into 1.2% agarose gels. They were tinted with ethidium bromide and visualized under an ultraviolet light trans-illuminator. Images thus obtained were documented with EDAS 290 system (Kodak, USA).
RESULTS
Figure 1 shows a typical example of bicuspids which had been previously subjected to bracket placement (experimental site group) to induce orthodontic tension during 1, 3, 5, 7 and 9 days. The image shows the upper right bicuspid where 30° angulation with respect to occusal plane can be appreciated. During the study of both groups: control group bicuspids, corresponding to zero time and experimental bicuspids, no noticeable clinical changes in periodontal ligament thickness could be observed during the time during which orthodontic forces were applied.
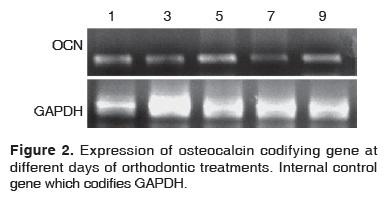
Figure 2 shows the expression of the osteocalcin codifying gene which presents a molecular weight of approximately 310 bases pairs. Results obtained during the days when orthodontic forces were applied indicated that osteocalcin was expressed during all experimental times, and exhibited expression increase during treatment days five and nine. During treatment days 1, 3 and 7, expression was similar to time zero or control; day seven showed a slight decrease.
DISCUSSION
Results obtained in the present study indicate the fact that when applying orthodontic forces with brackets to bicuspids during a span of one to nine days a change was elicited in the expression of the gene which codifies osteocalcin in the periodontal ligament area. Since osteocalcin plays a role in resorption as well as in the deposition of mineralized matrix, results obtained in the present study confirm the regulating role osteocalcin plays in the processes of periodontiums extracellular matrixes. Expression of osteocalcin gene during experimental times can be attributed to induction of initial bone and matrix resorption of periodontal ligament collagen fibers, which is needed for tooth movement and for osteoclasts specific recruitment at the resorption site. Other studies confirm the fact that in animal models, local application of protein increases speed of orthodontic movements.9
Expression of osteocalcin codifying gene kept a growing expression during the time of orthodontic movements. On the 5th day, an increase was observed; this suggested the fact that resorption maximum peak was being reached. On the 7th day a recessive phase took place, and in the 9th day a significant expression was observed. All the aforementioned would indicate the fact that a mineralization process was initiated, since there was a protein involved in regulating calcium and hydroxyapatite concentrations. Osteocalcin expression, as shown in figure 2 hints that the matrix of the periodontal ligament can be remodeled from the first hours in which brackets are applied. Scientific literature counts with several reports demonstrating the effect of orthodontic forces on the periodontal ligament. Nevertheless, these studies have been conducted in vitro and/or in species different from human beings.10
These first response stages of the periodontal ligament can be related to the inflammation processes elicited when inducing tooth movement with orthodontic processes. Therefore, osteocalcin could facilitate orthodontic movements by acting as a molecule which induces chemo-attraction to recruit osteoclasts at sites of greater pressure; it can also act as a key factor helping bio-mineralization locations at the tension site.
CONCLUSION
The present study suggested presence of changes at molecular level in the periodontal ligament matrix. These changes could be sensitive to movement induction time of the tooth when fixed appliances were used.
Molecular level changes were determined by the RT-PCR technique for the gene which codifies osteocalcin. This technique reflected the fact that the periodontal ligament matrix was sensitive, in a given time, to the orthodontic forces exerted on the teeth used in the present study.
Osteocalcin expression suggested double participation in orthodontic processes: it took part in resorption as well as remodeling processes of the periodontal ligament matrix.
REFERENCES
1. Bilezikian JP, Raisz LL. Principles bone biology. (USA): Academic Press; 1996. [ Links ]
2. Hauschka PV, Lian JB, Cole DEC, Gundberg CM. Osteocalcin and matrix gla protein-vitamin K-dependent proteins in bone. Physiol Rev. 1989; 69 (3): 990-1047. [ Links ]
3. Alcain FJ, Buron MI. Ascorbate on cell-growth and differentiation. J Bioenerg Biomembr. 1994; 26 (4): 393-398. [ Links ]
4. Geneser F. Histología. 3a edición, España, Ed. Panamericana. 2002. [ Links ]
5. Glowacki J, Rey C, Cox K, Lian J. Effects of bone matrix components on osteoclast differentiation (Review). Connect Tissue Res. 1989; 20 (1-4): 121-129. [ Links ]
6. Ducy P. Increased bone formation in osteocalcin deficient mice. Nature. 1996; 382 (6590): 448-452. [ Links ]
7. Xiao G, Jiang D, Gopalakrishnan R, Franceschi RT. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem. 2002; 277 (39): 36181-36187. [ Links ]
8. Kobayashi Y, Takagi H, Sakai H, Hashimoto F, Mataki S, Kobayashi K. Effects of local administration of osteocalcin on experimental tooth movement. Angle Orthod. 1998; 68 (3): 259-266. [ Links ]
9. Hashimoto F, Kobayashi Y, Mataki S, Kobayashi K. Administration of osteocalcin acelerates orhodontic tooth movement induced by close coil spring in rats. Eur J Orthod. 2001; 23 (5): 535-45. [ Links ]
10. Takano-Yamamoto T, Takemura T, Kitamura Y, Nomura S. Site-specific expression of mRNAs for osteonectin, osteocalcin, and osteopontin revealed by in situ hybridization in rat periodontal ligament during physiological tooth movement. J Histochem Cytochem. 1994; 42 (7): 885-896. [ Links ]
Note This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam Mailing address:
Mailing address:
Francisco Javier Marichi Rodríguez
E-mail: fmarichi@fo.odonto.unam.mx











 texto en
texto en 


