Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista odontológica mexicana
Print version ISSN 1870-199X
Rev. Odont. Mex vol.16 n.1 Ciudad de México Jan./Mar. 2012
Original research
In vitro adhesion of Candida albicans in three different tissue conditioners used in prosthodontics
Yasmín Bonilla Rodríguez,* Víctor Moreno Maldonado,§ Bertha Muñoz Hernández,II Gabriel Palma Cortés¶
* Graduate of the National School of Dentistry, National University of Mexico, UNAM.
§ Master in Dental Sciences, (Coordinator of the Prosthodontics Department of the National School of Dentistry, National University of Mexico (UNAM).
II Responsible for the Medical Mycology Department, Research Department, Instituto Nacional de Enfermedades Respiratorias (National Institute of Respiratory Diseases).
¶ Medical Science Researcher, Mycology Department, Instituto Nacional de Enfermedades Respiratorias (National Insitute of Respiratory Diseases).
ABSTRACT
Background: Tissue conditioners are materials used on the surface of denture bases for 4 to 7 days. These materials have an irregular surface, and if improperly used, there is the possibility of adherence of some microorganisms such as Candida albicans which could elicit Oral Candidiasis. Objective: To quantify and analyze three tissue conditioners with the aid of an Optical Microscope (OM) as well as a Scanning Electron Microscope (SEM) at three inoculation phases (days). Study: 36 study samples were prepared through inoculation of in vitro Candida albicans at 24, 72 and 168 hour intervals. Samples were assessed through OM and SEM from which photographs were obtained at 500x, 2,000x and 5,000x magnifications. Results: Coe Comfort tissue conditioner presented an even surface with budding yeast adhesion and hyphae; Lynal presented a 3 µ cracked surface at 72 and 168 hours with budding yeast; Flexacryl presented a 4 µ cracked surface at 72 and 168 hours and great amounts of budding yeasts. Conclusions: Coe Comfort conditioner presents a more regular surface with less Candida albicans adhesion, whereas Flexacryl is the one presenting greater irregularities and Candida albicans adherence in its surface.
Key words: Candida albicans, adherence, scanning electron microscope, optical microscope, oral candidiasis.
INTRODUCTION
Complete dentures, per force, must have a design which enables them to distribute all loads exerted upon them. The biomechanical aspect of complete dentures is based on their functioning and on three principles: retention, support and stability. When one of these factors is lost due to some external cause, the denture shows poor fit, and consequently there is resorption of the residual ridge, pain, discomfort and inflammation of the tissue directly related to the prostheses. Also to be taken into account are the facts that the denture is in constant use, not allowing therefore tissue regeneration, equally to be taken into account is the possibility of poor hygiene on the ridges and on the prostheses; due to all these factors, the patient faces an inevitable reaction of the denture supporting tissues.1,2,4,5,10
Many of these tissue reactions can be reversed if the cause causing the damage is alleviated. Nevertheless, it is generally necessary to treat the area which will be supported by the denture with tissue material or tissue conditioner. Several weeks might be needed to revert tissue damage.4-6
Tissue conditioners are resilient plastic materials (viscoelastic) which flow and intimately adapt to the tissue mucosa and the base of the oral prosthesis. They function as a cushion and absorb part of the energy elicited by the impact of mastication between oral tissue and denture surface, they equally provide a relief zone to irritated or damaged tissue.7,15,16 Some irregularities (porosities) are present on the conditioners surface; they favor adherence of several microorganisms and form a microbial plaque mainly composed of bacteria and yeasts.
Tissue conditioners are of temporary usage (5-7 days). After this period they begin to lose elasticity and softness, and become irritants as they turn rough, hard, and defective. These defects can act as reservoirs which contribute to the adherence and proliferation of microorganisms. Candida albicans is the microorganism most frequently isolated in patients wearing dentures.12,24,25
Candida albicans is a microscopic, oval, yeast shaped, thin walled fungus measuring from 2 to 4 µm. Nevertheless, in infected tissues, filamentous shapes of variable length have been identified having 3 to 5 µm rounded ends. Pseudohyphae have also been identified (elongated yeast cells which remain bonded together). Candida albicans is a pathogenic, opportunistic agent, causing severe infectious processes in the oral cavity. Oral candidiasis is one of these infectious processes, it occurs in patients wearing dentures, in them, there is a thinning of the epithelial tissue in contact with the denture and conjunctive tissue is severely inflamed. It is generally located on the palate surface, affects more females than males, and elicits a constant burning awareness.20 Candida albicans proliferation and its adherence to prostheses (dentures) has been related to the onset, maintenance and aggravating of Oral Candidiasis.3,5-7,10,13,17,19
The initial phase of Candida albicans adhesion to the tissue conditioning is mediated by non specific factors (surface hydrophobia and electrostatic forces) as well as by cellular wall components (mannoproteins and fibrillar proteins) which act as adhesins and are able to recognize receptors in the epithelial cells, extracellular matrix and surfaces colonized by oral streptococci.21,24 Once adhered, the microorganism can reproduce, develop biofilms, change its type of growth from blastoconidia to pseudo hyphae and true hyphae. These can guide its growth through contact with the discontinuities of the oral mucosa cells, penetrate into them, invade deep tissues and hinder phagocytosis.5,17
The aim of this study was to quantify and analyze three tissue conditioners with the use of Optical Microscopy (OM) and Scanning Electron Microscopy, at three phases (times) of inoculation.
MATERIAL AND METHODS
Sample preparation: Thyirty six slides of tissue conditioners 10 mm long x 10 mm wide x 1 mm thickness were prepared. 12 slides were prepared with Coe Comfort, 12 slides with Lynal, and 12 slides with Flexacryl. Out of each 12 slide set, 6 samples were allocated to be observed under optical microscope, and 6 under MEB STEREOSCAM 440, with the aim of carrying out duplicate studies at three inoculation times (24, 72, and 168 hours) and applied according to manufacturer's instructions. Obtained samples were cleansed (washed) with sterile distilled water , and later disinfected for one hour with broad spectrum antibiotic, in this case 500 mg amikacin, to eliminate bacteria from the samples, leaving an optimum surface for Candida albicans adhesion.8
Handling of microorganisms and culture conditions: A Candida albicans reference strain was used, provided by the Institut Pasteur, France. This reference strain was kept in Sabouraud dextrose agar culture plates. This strain was subject to germ tube formation tests, clamidoconidia, so as to ascertain strain purity. The microorganism was cultivated in Sabouraud dextrose agar plates, at 37 °C during 24 to 48 hours. From this fresh culture, a microbiological batch was harvested, and it was incorporated to 100 mL of sterile tripticase soy broth, which was kept in an elliptical incubator for a whole night (12 hours) at 37 °C with 60 rpm agitation.9,19
The culture was centrifuged for 10 minutes at 1,000 rpm. Later, the tripticase soy broth was withdrawn, and the blastoconidia present in the sediment were twice washed with sterile saline solution. The washed sediment received 2 mL of sterile tripticase soy broth to re-suspend blastoconidia. This concentrate was gradually incorporated into 10 mL sterile tripticase soy broth up to the point where a 0.8 OD520 (1 x 107 blastoconidia/mL) suspension of the microorganism was obtained.20,21
Biofilm formation and adherence test: Samples were placed in 24 wells cellular culture plates with 2 mL of Candida albicans suspension. Samples were incubated during the following periods: 24, 72 and 168 hours at 37 °C with 6 rpm agitation. Once these periods elapsed, they were washed with PBS (NaCl 8 g, KCl 2 g, KH2PO4 2 g, KH2PO4 - 12H2O 2.89 g and 1 liter distilled H2O pH 7.4) to remove non adhered cells.7
Yeast growth determination and quantification was carried out with an optical microscope using Gram staining technique. To perform counting, a reticulum was set up in the ocular portion. This reticulum was divided into ten 1 mm fields wide by ten 1 mm fields long. In such a way yeast count (Gram positive) per mm2 was achieved.2,7
Determination of surface characteristics and structure was performed with MEB STEROSCAM 440, in such a way as to obtain photographic increases of 500x, 2,000x, and 5,000x. Samples were previously fixated with (2.5%) glutaraldehyde, buffer washed and dehydrated with 20%, 40%, 60%, 80% and 100% ethanol. Later they were mounted on brass pellets and ionized with a coating of gold-palladium during 3 minutes at 1,500 KV and 10 mA.7
Statistical analysis: Adhered cells quantification was carried out with the Sigma Star Ver 3.1 statistical package. A general descriptive statistic was performed for all variables, and adhered yeast averages were compared in the different tissue conditioners used, as well as inoculation time with a one way ANOVA. The Tukey test was used for data comparison purposes.
RESULTS
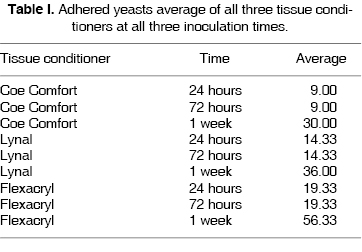
Data gathered showed that when using Coe Comfort tissue conditioner, after 24 hours with the use of OM, a 9 yeast per mm2 count was obtained, and with SEM a predominantly even surface was observed, with absence of porosities, and yeasts measuring from 3 to 3.5 µm in diameter, well circumscribed and in budding process (Figure 1-A). After 72 hours, in the OM a 9 yeasts per mm2 count was obtained, and with SEM the surface was observed with 50 to 60 µm and with 3.5 to 4 µm yeasts in budding state (Figure 2-A). After 168 hours, with optical microscope a 30 yeast per mm2 count was found and with electron scanning microscope a surface with depressions and yeasts in hyphae state were observed (Figure 3-A).
When using Lynal tissue conditioner, after 24 hours, with OM, a count of 14 yeasts per mm2 was obtained, and with SEM a predominantly even surface was observed, with scant irregularities and 3 to 3.5 µm diameter, well circumscribed and budding state yeasts (Figure 1-B). After 72 hours, OM showed a count of 14 yeast per mm2 and with SEM the surface presented 2 to 3 µm thick irregular cracks and 3 to 3.5 µm diameter, budding state yeasts inside the sample cracks (Figure 2-B). After 168 hours, with the OM a count of 36 yeasts per mm2, and the SEM showed a surface with 3 µm thick cracks and yeast measuring from 3 to 3.5 µm in diameter, well circumscribed and in budding state (Figure 3-B).
When using Flexacryl tissue conditioner, after 24 hours and with OM, a count of 19 yeasts per mm2, and with SEM an even and regular surface was observed, with very few irregularities, with 3 to 3.5 µm diameter yeasts, well circumscribed, in budding state (Figure 1-C). At 72 hours, with OM a 19 yeast per mm2 count was obtained, and with SEM a mostly irregularly creviced surface was observed (2 to 4 µm crevices) with 3 to 3.4 µm diameter yeasts, in budding state, observed in the junction (union) of the sample s crevices (Figure 2-C). After 168 hours, with OM a 56 yeasts per mm2 count was observed, and the SEM revealed a surface with 3 to 4 µm thick crevices, well circumscribed, in budding state and abundant throughout the whole surface (Figure 3-C).
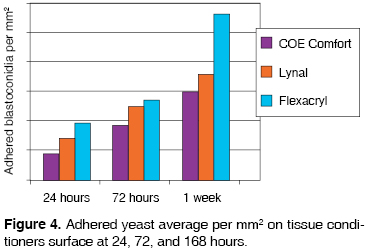
With the help of gathered data, it was observed that statistically, there are variations in adherence (p ≤ 0.05) when comparing Flexacryl and Coe Comfort tissue conditioners at 24, 72 and 168 hours, and between Flexacryl and Lynal at 168 hours, which gives a result of Flexacryl 24 hours (19.3 ± 3.055) and Coe Comfort 24 hours (9 ± 3.6); Flexacryl 72 hours (19.3 ±3.055) and Coe Comfort 72 hours (9 ± 3.6); Flexacryl 168 hrs ( 56 ± 2.517) and Lynal 168 hrs (36.6 ± 1.0); Flexacryl 168 hrs (56.3 ± 2.517) and Coe Comfort 168 hrs (30.0 ± 2.0). All the aforementioned data are presented in table I and figure 4.
DISCUSSION
According to previous studies where different tissue conditioners were compared at same inoculation times but with different brands (Visco-gel, Fixo-gel, Fitt,23 Coe Comfort, Coe-Soft, GC soft),22 MJ King & al22 and Kulak Y & al23 observed that Candida albicans adherence to these samples was immediately present after 24 hours of the inoculation. There was adherence of Candida albicans one hour after inoculation. This shows that adherence is influenced by the structure of the material. This agrees with the present study where it was demonstrated that materials, with time, experience important alterations which cause damage in the oral tissue; for this reason it must be constantly changed.
In the present study, as well as in that of Kulak Y & al,23 MJ Kim & al22 it was observed that tissue conditioners, when in contact with Candida albicans, and with time, favor the adherence of the aforementioned fungus, but, depending on the composition of each conditioner, adherence varies. In the present study it was observed that the greater the deterioration of the material, greater would be the fungal adherence.
This study concurs with other authors with respect to observing the importance of studying the biofilms7,8,12,14-16,22-23 formed by Candida albicans with the aid of a SEM. In our days this method has proven to be most trustworthy, valuable and practical to allow observation of the sample surface, as well as the architecture of the microbial communities established on the aforementioned structure.
CONCLUSIONS
Based on results obtained in this study it can be concluded that the observed sample surface characteristics showed defects in each and every one of the tissue conditioners tested. These defects increased with the passing of time, since the surfaces became hard and rough, crevices were observed, as well as depressions and irregularities.
The conditioner Coe Comfort presented a smoother surface and with lesser Candida albicans adherence. For this reason, no statistically significant growth data were gathered.
The conditioner Flexacryl presented, on its first 24 hours an even surface with few imperfections, but, with the passing of time, the sample surface became very irregular, with 3 mm thick crevices which promoted greater Candida albicans adherence. This resulted into this material having greater blastoconidia amounts, and presented statistically significant growth data at 24, 72, and 168 hours.
ACKNOWLEDGEMENTS
We wish to acknowledge assistance granted by the Prosthodontics interdisciplinary laboratory where the samples were taken, to the National Institute of Respiratory Diseases Medical Mycology Laboratory where the microbiological development and yeast count of this study were undertaken, and to the Materials Research Institute of the National University of Mexico, (UNAM), where the STEROSCAM 440 Scanning Electron Microscope analysis was carried out.
REFERENCES
1. Winkler S. Prostodoncia total. 1a ed. México, Editorial Limusa, 1999. [ Links ]
2. Zarb, GA, Hickey JC, Bolender CI, Carlsson G. Prostodoncia total de Boucher. 10a ed. México, Editorial Interamericana; 1990. [ Links ]
3. Pardi G. Determinantes de patogenicidad de Candida albicans. Acta Odontológica Venezolana 2002; 40 (2): 185-192. [ Links ]
4. Quiroga R. Acondicionador de tejidos . Universidad de Mayor. Fac. de Odontología, 2002: 1-14. [ Links ]
5. Serrano GC. Estudio in vitro de la adherencia de Candida albicans a las resinas. Univ. Complutense de Madrid, 2002; 127: 7-8, 19-20. [ Links ]
6. Pardi G, Cardozo EI, Perrone M, Salazar E. Detección de especies de Candida en pacientes con estomatitis subprotésica. Acta Odontológica Venezolana, 2001; 39 (3). [ Links ]
7. Rostoka D, Krocha IU, Kuznetsova V, Renis A, Tremane R, Uikovskaia T, Vanka A. Candida albicans adhesion to plastics during correction of removable dentures. Stomatologia (Mosk) 2004; 83 (5):14-6. [ Links ]
8. Romo AE. Análisis microscópico de la adherencia de Candida albicans in vitro sobre resina acrílica utilizada para bases de dentaduras procesadas con tres diferentes técnicas Revista Odontológica Mexicana 2006; 10 (4): 167-172. [ Links ]
9. Wright PS, Young KA, Parker S, Kalchandra S. Evaluating the effect of soft lining materials on the growth of yeast. J Prosthet Dent 1998; 79: 404-409. [ Links ]
10. Cal E, Kesercioglu A, Sen BH, Cilli F. Comparison of the hardness and microbiologic adherence of four permanent denture soft liners. Gen Dent 2006; 54 (1): 28-32. [ Links ]
11. Nikawa H, Taizo H. Interactions between thermal cycled resilent denture lining materials, salivary and serum pellicles and Candida albicans in vitro : Part II Effects on fungal growth. J Oral Rehabil 2000; 27: 124-130. [ Links ]
12. Nikawa H, Egusa H. Alteration of the coadherence of Candida albicans with oral bacterial by dietary sugars. Oral Microbiol Immunol 2001; 16: 279-284. [ Links ]
13. Nevelainen M, Nari T. Oral mucosal lesions and oral hygiene habits in the home-living elderly. J Oral Rehabil 1997; 24: 332-337. [ Links ]
14. Bulad K, Taylor RL, Verran J, McCord JF. Colonization and penetration of denture soft lining materials by C. albicans . Dent Mat 2004; 20: 167-175. [ Links ]
15. Liébana UJ. Microbiología oral. Características generales de los hongos patógenos humanos . 1a ed. Madrid, Mc Graw Hill Interamericana de España; 1995: 1362-75. [ Links ]
16. Rodríguez OJ, Miranda TJ, Morejón LH, Santana GJC. Candidiasis de la mucosa bucal. Revisión bibliográfica Rev Cubana Estomatol 2002; 39 (2): 187-233. [ Links ]
17. Gordon R, Stephen SP. Biofilm of Candida albicans. A update. Eukaryotic Cell 2005; 4 (4): 633-638. [ Links ]
18. Waltimo T, Johanna T. Adherence of Candida albicans to the surface of polimethylmethacrilate-e glass fiber compositeused in dentures. Int J Prosthodont 1999; 12: 83-86. [ Links ]
19. Adam B, Baillie GS. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis . J Med Microbiol 2002; 51: 344-349. [ Links ]
20. García-Rodríguez JA, Picazo JJ. Microbiología médica general . Madrid: Ed. Mosby Doyma; 1996: 625-628. [ Links ]
21. Bermejo FA. Medicina bucal Vol. I. Madrid, Ed. Síntesis S.A. 1998: 139-51. [ Links ]
22. Kulak Y, Kadir T. In vitro study of fungal presence and growth on three conditioner materials: J Marmara Univ Dent Fac 1997; 2 (4): 682-4. [ Links ]
23. Kim MJ, Shin SW, Lee JY. In vitro study on the adherence and penetration of Candida albicans into denture soft lining materials. J Korean Acad Prosthodont 2006; 44 (4): 466-476. [ Links ]
24. Waters MGJ, Williams DW, Jagger RG, Lewis MAO. Adherence of C. albicans to experimental denture soft lining materials. J Prosthet Dent 1997; 777: 306-312. [ Links ]
 Correspondence address:
Correspondence address:
C.D. Yasmín Bonilla Rodríguez
Calle Morelos Núm. 13-Int. 10, Col. Emiliano Zapata
Delegación Coyoacán 04815, México, D.F.
Telephone number: 56840070
E-mail: yasboni@msn.com
o yasboni@hotmail.com
Note
This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam











 text in
text in 


