Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Tropical and subtropical agroecosystems
versión On-line ISSN 1870-0462
Trop. subtrop. agroecosyt vol.13 no.1 Mérida ene. 2011
Artículos de investigación
Assessment of Trichoderma isolates for virulence efficacy on Fusarium oxysporum F. sp. Phaseoli
Evaluación de la eficacia de aislamientos de Trichoderma sobre Fusarium oxysporum F. sp. Phaseoli
Jane A. Otadoh1, Sheila A. Okoth2, James Ochanda2 and James P. Kahindi3
1 School of Biological Sciences.
2 Center of Biotechnology and Bioinformatics, University of Nairobi, P. O. Box 30197- 00100, Nairobi, Kenya.
3 United States International University, P.O.Box 14634 - 00800, Nairobi Campus.
Email: Akinyijao2000@yahoo.com
* Corresponding author
Submitted April 12, 2010
Accepted May 25, 2010
Revised received July 20, 2010
Abstract
Trichoderma has been widely studied for their biocontrol ability, but their use as biocontrol agents in agriculture is limited due to the unpredictable efficiency which is affected by biotic and abiotic factors in soil. Isolates of Trichoderma from Embu soils were evaluated for their ability to control Fusarium oxysporum f. sp. phaseoli., in vitro and promote seedling growth in the greenhouse. Bioassays were run using dual cultures and diffusible compound production analysis. The Trichoderma isolates significantly (p ≤ 0.01) reduced the mycelial growth of the pathogen. The principle mechanisms of niche competition, mycoparasitism, and antibiosis were observed in growth of the pathogen mycelium in the presence of Trichoderma spp., through development of inhibition zones. There was coiling of hyphae around the pathogen mycelium coupled by lysising of cell wall Trichoderma spp., where T. reesei and T. koningii were the most effective isolates. Studies were indicative of the synergistic ability of Trichoderma spp., being an effective biocontrol of bean seedlings against Fusarium wilt while also promoting plant growth.
Key words: Fusarium oxysporum f. sp. phaseoli.; Trichoderma spp.; Mycoparasitism inhibition; Fusarium wilt.
INTRODUCTION
Soil borne plant pathogens cause economic losses annually in many crops hence on food security. Wilt caused by Fusarium oxysporum, a soil borne pathogenic fungi which is considered as one of the constraint responsible for 50-100% crop losses resulting in low productivity Haware and Nene, (1982, 1980) due to early wilting. Control of Fusarium by chemicals is often uneconomical and has negative environmental impacts and development of fungicidal resistance variants. Biological management is considered an environmentally acceptable alternative to existing chemical treatment methods for control of soil pathogenic fungi causing wilt (Harman et ai, 2004, Eziashie et al., 2007).
Embu district, which is the area of study, was characterized by intensive management of agro-ecosystems through use of high level inputs such as fertilizers and pesticides for food and cash crops. Beans (Phaseolus vulgaris L.) is a widely grown legume and often intercropped (Okoth et al 2010) with maize (Zea mays) and yield losses by Fusarium spp. are estimated to be 80%, Thung and Rao(1999). Trichoderma spp are known to have biological mycoparasites and which are used commercially as biocontrol against a range of plant pathogenic fungi such as Fusarium, Pythium, and Rhizoctonia strains as well as a product of ecological interest (Mausam et al., 2007).
The study was conducted to screen and evaluate locally isolated Trichoderma spp. in controlling Fusarium oxysporum f. sp. phaseoli., and evaluate their efficacy in screenhouse.
MATERIALS AND METHODS
Isolation and identification of Trichoderma spp.
Trichoderma spp. were isolated from soil collected from farmer field in Embu and Taita Districts in Kenya with plants showing Fusarium wilt symptoms. Soil dilution plate technique which is described by Johnson et al., 1959 and soil washing methods (Bills & Polishok 1994; Gams et al., 1987) was used for dilution process. To obtain the fungi from the soil, cultures were grown on malt extract agar (MEA) and cornmeal agar (CMA) with 2% dextrose, both with streptomycin 50mg/l and cyclosporine 10mg/1 was used (Foogle et al 2007). After 4 weeks of growth colonies were counted, identified then transferred to Petri dishes containing potato dextrose agar (PDA) and incubated at 15, 30 and 35° C for further identification to species level. Genus identification of green fungus was done using the method of Domsch et al., (1980). Trichoderma isolates were identified to species level following the taxonomic key of Samuels et al., (2004). Microscopic examination was carried out by mounting the culture in lactophenol cotton blue but for size measurements KOH and water was used as the mounting fluid. A small amount of material was placed in a drop of 3% KOH on a slide and then replaced with water. The isolates were preserved in sterile soil and stored at 4° C
Isolation of Fusarium oxysporum f. sp. phaseoli, from diseased bean plant tissues
Diseased bean plant tissues were obtained from the University of Nairobi farm at Collage of Agriculture and veterinary medicine (CAVS) and oxysporum f. sp. phaseoli., were obtained. The plant tissues were washed under running tap water to remove surface soil, dust and other contaminants. Tissue pieces were cut out from the leading edges of lesions and placed in 1% sodium hypochloride for five minutes then rinsed in sterile distilled water and dried on sterile filter paper. The dried pieces were cut into smaller pieces, plated onto PDA and incubated at 25° C
Antagonistic isolates of T. koningii, T. asperellum, T. atroviride, T. reesei, and T.harziunum, against Fusarium oxysporum f. sp. phaseoli were tested. Trichoderma isolates were evaluated for their antagonistic activity against Fusarium oxysporum f. sp. phaseoli., in vitro following the dual culture technique (Odebode and Sobowale 2001; Skidmore and Dickinson, 1976). The culture plates were incubated for six days at 25°C, colony growth of both biocontrol agents and pathogen were observed constantly and the radial growth of the pathogen recorded daily upto day four of inoculation, the percent inhibition was worked out as follows:
PI = C-T x 10/ C
Where:
PI = Percent inhibition of mycelial growth,
C = Radial growth of pathogen in control plates (cm),
T = Radial growth of pathogen in dual culture (cm).
Cultures were maintained on PDA slants at 4°C. The experiment had six treatments with three replicates.
Experiment 1: Antagonism test of Trichoderma spp. against Fusarium oxysporum f. sp. Phaseoli., on culture Plates
In order to select the most efficient isolates of Trichoderma against Oxysporum f. sp. phaseoli the local isolates were subjected to a further in vitro screening. The pathogen was inoculated on sterilized PDA and grown for seven days. After the establishment of the pathogen in the Petri dishes, a 5 mm culture disc of the antagonist was inoculated. The plates were kept at room temperature for nine days. Each set of treatment was replicated three times. The plates were observed daily and growth antagonism ratings were made using the modified Bell et al. (1982) scale (Classes 1 to 5) as follows; Class 1 = the antagonist completely overgrown the pathogen (100 % overgrowth). Class 2 = the antagonist overgrown at least 3/4th of pathogen surface (75% overgrowth). Class 3 = the antagonist colonized on half of the growth of the pathogen (50% overgrowth). Class 4 = the pathogen and the antagonist locked at the point of contact. Class 5 = the pathogen overgrown the mycoparasite
Experiment 2: Antagonism test of Trichoderma spp. against Fusarium oxysporum f. sp. Phaseoli., on culture slides
A clean slide was placed on a Z-shaped glass rod in a 9-cm-diameter petri dish and autoclaved. A small amount of molten water agar was poured and evenly spread over the slide to make a thin agar film. One end of the slide was kept free of the medium to facilitate handling. The inocula of T. reesei and the pathogen were placed on the slide one cm apart from each other. One ml of sterile water was added to the petri dish to prevent drying and incubated for seven days at 25°C. At the end of the incubation period, regions where the hyphae of T. reesei, met the hyphae of the pathogen were observed under a light microscope for the presence of coiling structures or wall disintegration following the technique of Sivakumar et al., (2000). The treatments were replicated three times.
Experiment 3: Suppression of Fusarium oxysporum f. sp. PhaseolL, by Trichoderma spp., in the greenhouse
Common bean was used as the test crop for conducting the bioassay under greenhouse conditions. The four treatment set were; Control which was the uninnoculated seed, seed with pathogen oxysporum f. sp. phaseoli., inoculum, seed innoculated each with T. koningii, and T. reseei inoculum. The Pathogens were obtained from fourteen day old cultures grown on Potato Dextrose Agar (PDA) plates incubated at 250C room temperature. Microconidia between 107 and 1010 colony forming units (Mausam, 2007) were prepared by serial dilution of fungus with sterile distilled water. Conidial densities in the suspension were determined by use of hermocytometer under a light microscope. 20 mycelial discs (5mm) of Trichoderma isolates and 10 mycelial discs (5mm) of oxysporum f. sp. Phaseoli., cut from the agar were used as soil inoculum (Ghildiya, 2008)
Treatments to evaluate Trichoderma spp for virulence efficacy on Fusarium oxysporum f. sp. Phaseoli., in screenhouse
Treatments were conducted in Plastic pots (10 cm diameter) containing one kg of peat soil previously autoclaved at 121°C for 1 hr on three successive days and filled to 2/3 of pot volume. In the first treatment, a mixture containing 10 mycelial discs (5mm) of approximately 1.6xlO6 colony forming units (cfu) of the pathogen oxysporum f. sp. Phaseoli., alone was prepared. In the second and third mixture the treatments contained autoclaved soil with cow dung manure and mavuno fertilizers respectively. The fourth and fifth pot each contained a mixture of soil and T. reseei with manure and mavuno respectively. The sixth treatment contained 20 mycelial discs (5mm) approximately 3.01xl08 colony forming units (cfu) of T. koningii, mixed thoroughly with 10 mycelial discs (5mm) approx 1.6xlO6 colony forming units (cfu) of oxysporum f. sp. Phaseoli. The seventh and the last treatment contained soil mixture each with T. reseei alone and uninoculated seed as control. The treatments were repeated with 20 mycelial discs (5 mm) approx.5.6xl07 colony forming units (cfu) of T. koningii. Each plastic pot was seeded with two bean seeds (Phaseolus vulgaris L.). The experimental design was completely randomized with two replicates (pots) for each Treatment. The greenhouse temperatures of were maintained between 25°C and 26°C. Plants were harvested after three weeks from seeding and all seedlings uprooted and evaluated. Phenotypic data were obtained by infecting bean seedlings with oxysporum f. sp. Phaseoli.
To test antagonism effect of Trichoderma on the pathogenic fungus, promising isolates of T. koningii, and, T. reesei earlier screened were selected for evaluation against Fusarium rot, using a rating scale of 0-5 described by Filion et al., (2003). Disease severity was assessed at 21 days after inoculation. Seedlings were removed from the pots and excess soil clumps were removed by gently shaking and dipping the roots in water. Roots were dried with a paper towel and rated immediately for symptoms of root rot. The severity of root rot was visually scored by assessing necrotic lesions on the roots and hypocotyls using a rating scale of 0-5 (Abeysinghe, 2007) as follows; 0= no disease symptoms, 1= slightly brown <50% surface discoloration of the hypocotyls, 2= >50% surface discoloration, 3= discolored hypocotyls, <50% surface discoloration of the hypocotyl, 4= darkly discolored hypocotyls and root completely collapsed and severe root pruning, 5= dead or dying plants.
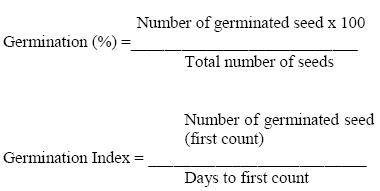
Germination response
Germination of inoculated seeds was compared with untreated control 14 days after sowing. Germination percentage and germination index of bean seeds were calculated according to Irum Mukhtar, (2008) as follows;
All the experiments mentioned in this paper were laid out in completely randomized design and the data were subjected to Analysis of Variance (ANOVA) and means separated using Duncan's multiple range tests.
RESULT
Inhibition of mycelial growth of Fusarium oxysporum f. sp. Phaseoli
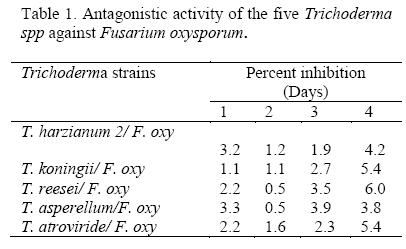
All Trichoderma isolates that were screened inhibited growth of F. oxysporum f. sp. Phaseoli (p ≤ 0.01) as their biocontrol potency increased with time. At 96 hours after inoculation all the biocontrol agents exhibited strong (5) to very strong antagonism (6) on an assessment scale of 0 to 6 (Table 1). T. reesei was the most effective among Trichoderma species that were tested.
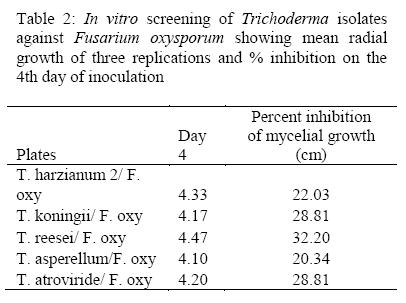
The highest inhibitory effect on growth of pathogen oxysporum f. sp. Phaseoli was achieved by T. reesei (32.2) followed by followed by T. atroviride and T. koninngii both at 28.8 while isolate of T. asperellum showed the lowest inhibitory effect to Fusarium oxysporum f. sp. Phaseoli (20.3) as shown in Table 2. According to these results, T. reesei and T. koninngii were used in the further experiments.
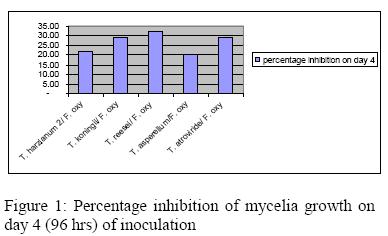
Considerable variations in the inhibitory properties of Trichoderma isolates were discernible when assessed on the 4th day of inoculation (Figure 1). Variations in the inhibitory potential may be due to the differences in the quantity and quality of the inhibitory substances produced by the antagonists which agrees with Hassan et al. (2008)
All Trichoderma antagonistic interactions on F.oxysporum f. sp. phaseoli., reduced the radial mycelia, T. reseei overgrow the pathogen. It was also observed that T. reesei and T. koningii were more effective against oxysporum f. sp. phaseoli than other Trichoderma strains at day seven (Table 3)
Trichoderma hyphae was observed to coil around the fungal mycelium which was followed by cell wall degradation and cellular coagulation of the pathogen hyphae, and T. reseei and T. koningii were the most effective which agreed with Howell et al (2004).
The five Trichoderma isolates tested in vitro were effective in suppressing oxysporum f. sp. Phaseoli ( p ≤ 0.05). Growth suppression by T. reesei against oxysporum f. sp. Phaseoli had the highest effect in inhibition of mycelial growth with p-value (0.018), followed by T. koningii (p=0.014), T. asperellum (p=0.013), T. harzianum (p=0.012) and T. atroviride (p=0.012) respectively, meaning that the isolates were effective in controlling fungal mycelial growth in bean seedlings (Figure 2)
Efficacy of Trichoderma spp on Fusarium wilt disease of bean in the greenhouse
Bean plants treated with Trichoderma isolates against. F. oxysporum f. sp. phaseoli., were free of Fusarium wilt. Reduced lesions and brown discolorations were observed in bean root inoculated with T. reesei and by T. koningii. Pathogen inoculated control expressed brown to dark discolorations. There was reduced root mass with loss of lateral root branching as shown by the arrows (Plate 3a). Plants infected with Fusarium root rot displayed stunted and reduced root systems, Burke and Hall, (1991). Plants treated with T. reesei and T. koningii singly and the control with no treatment showed no signs of root wilt (Plate 3b)
Disease severity was visually scored by assessing the lower hypocotyls discoloration using a modified Filion (2003) rating scale of 0-5. All bean seedlings grown in soil inoculated with a conidial suspension of F. oxyspurum f. sp. phaseoli had a mean score rating of 3.63 of infection showing red lesions on hypocotyls and tap roots characteristically distinctive of Fusarium root rot. In comparison, plants from seeds treated with Trichoderma isolates against. F. oxysporum f. sp. phaseoli., conidia showed less number of lesions and low disease severity. The non-infested controls showed no symptoms while T. reesei treated plants were more protected (1.06) from the pathogen infection than T. koningii at a rating of 2.18 (Table 4) as reported by Chaudhary et al. (2006).
Promotion of growth of bean seedling responding to inoculation with Trichoderma species
In this study, Trichoderma isolate treatments enhanced bean root growth under greenhouse conditions although the degree of growth promotion varied with treatment. Harman, (2006) and Manju and Mall, (2008) have reported positive observation of Trichoderma species which increased plant growth and productivity. The results showed that there was a significant increase in bean seedling's emergence, fresh and dry root weights, three weeks after sowing. Bean seedlings treated with T. koningi and T. reesei isolates increased in root dry weight by 1.4 to 1.8gm and root fresh weight by 1.4 to 1.5 respectively (Figure 2) which is supported by Barakat et al (2007).
Trichoderma isolates exhibited an ability to compete for key exudates from seeds that stimulated the germination of propagules of plant pathogenic fungi in soil as they competed with microorganisms for nutrient and space. In this case, the germination of seed were all better than the control with a germination index of 1.86 for T. koningi and 2.29 for T. reesei. Enhanced seed germination by Trichoderma species have been reported by Mukhtar (2008).
DISCUSSION
Based on the dual culture, all Trichoderma isolates overgrew the oxysporum f. sp. phaseoli colonies degrading its mycelium. Similar results were observed by Barakat et al., (2006; 2005). Among the five fungal isolates, two namely T. koningii, and T. reesei, were promising biocontrol agents against oxysporum f. sp. phaseoli as they arrested the growth of the pathogen. T. reesei inhibited the growth of oxysporum f. sp. phaseoli and controlled the production of conidia due to its antagonistic properties against the pathogen. Trichoderma spp. biocontrol potential is as a result of a number of qualities which include antagonism, antibiotics and degrading enzymes which digest the cell wall, and similar observations were reported by Kamal (2009) and Harán et al., (1996). Diffusible substances inducing morphological abnormalities in the fungal mycelia and host cell contents have been linked with disorganization and deformities in mycelia hypha, and conidial structures as observed with T. reesei and T. koningii. This activity could also be attributed to the ability of Trichoderma spp to develop direct exchanges with pathogens to produce antimicrobial substances since mycoparasitism involved physical, coupled by synthesis of hydrolytic enzymes and antibiotics as reported by (Radwan, 2007).
Application of Trichoderma isolates as a conidial suspension reduced disease severity caused by oxysporum f. sp. phaseoli. Reduction in disease incidences and protection of bean seedling against Fusarium wilt was significant with variability within the isolates with T. reesei testing stronger ability in controlling oxysporum f. sp. Phaseoli compared to T. koningii The antagonistic ability of Trichoderma spp, has been reported as highly variable (Chet, 1990), as is shown in this study both in vitro and in the screen house. The reduced Fusarium wilt on beans may have been due to reduced population density of the pathogen, an observation which is supported by Bisht et al. (2008). The principle is related to the antagonistic properties of Trichoderma which involve parasitism and lysis of pathogenic fungi and competition for limiting factors in the rhizosphere mainly iron and carbon as reported by Sivan and Chet, (1986). This demonstrated ability of Trichoderma spp, to control the pathogen that cause wilt disease, are also been reported to contribute to ability to induce plant growth and development in greenhouse (Vinale et al., 2004).
It was also revealed that bean seedlings planted in Trichoderma isolate treated soils increased growth root dry and fresh weight and enhanced seedling emergence. Investigations suggest that the increased growth response caused by Trichoderma isolates may be through modifiacation of the rooting system as Yedidia et al. (1999) reported for T. harzianum inoculation which improved uptake of nutrients by the plant at a very early growth stage. Furthermore, Harman (2000) established that Trichoderma spp, are opportunistic plant colonizers that affect plant growth by promoting abundant and healthy plant roots, possibly via the production or control of plant hormones. Similar response has been explained (Kleifeld and Chet, 1992) as due to the ability of Trichoderma spp, to inhibit minor pathogens in the rhizosphere which might induce seed rots and pre-emergence damping off.
In conclusion, the study demonstrated that local isolates of T. reesei and T. konongii have potential for use as biological control agents to protect bean plants from oxysporum f. sp. Phaseoli. However, further studies on utilization of local isolates of biocontrol agents and evaluation of these Trichoderma isolates under field conditions is recommended.
ACKNOWLEDGEMENTS
The authors are sincerely thankful to the kenyan belowground biodiversity (BGBD) team for financial and intellectual support. The views and opinions expressed in this article are those of authors. Last but not the least, the authors would also like to thank professor Karanja (University of Nairobi, Collage of Agriculture and veterinary medicine) for her valuable suggestions to this work.
REFERENCES
Bell, D.K., Wells, H.D. and Markham, C.R. 1982. In vitro antagonism of Trichoderma spp. against six fungal plant pathogens. Phytopathology, 72: 379-382. [ Links ]
Campbell, R. 1989. Biological Control of Microbiol Plant Pathogens. Cambridge University Press, Cambridge, 432p. [ Links ]
Chaudhary S. A.R. Anderson, S.J. Park and K. Yu, 2006., Comparison of screening methods for Resistance to Fusarium Root Rot in common Beans (Phaseolus vulgaris L) Burke DW, Hall R.Fusarium root rot. In: Hall R (eds), Compendium of Bean Diseases. St. Paul, Minnesota, USA, APS Press, 1991, pp. 9-10. [ Links ]
Sivakumar D, R.S. Wilson Wijeratnam, R.L.C. Wijesundera, EM.T. Marikar and M. Abeyesekere., 2000. Antagonistic Effect of Trichoderma harzianum on Postharvest Pathogens of Rambutan (Nephelium lappaceum). Phytoparasitica. 28: 240-247. [ Links ]
Dennis, C. and Webster, J. 1971. Antagonistic properties of species groups of Trichoderma II. Production of volatile antibiotics. Transactions British Mycological Society, 57: 41-48. [ Links ]
Filion, M, St-Arnaud, M, Jabaji-Hare, S. H. 2003 Quantification of Fusarium solani f. sp. phaseoli in mycorrhizal bean plants and surrounding mycorrhizo sphere soil using realtime polymerase chain reaction and direct isolations on selective media. Phytopathology 93:229-235. [ Links ]
Ghildiyal A. and Pandey A. 2008. Isolation of cold tolerant Antifungal strains of Trichoderma sp from glacial sites of Indian Himalayan Region. Research Journal of Microbiology. 3: 559- 564. [ Links ]
Hanson, L. E. and Howell, C. R. 2004. Elicitors of plant defense responses from biocontrol strains of Trichoderma virens. Phytopathology 94:171-176. [ Links ]
Irum Mukhtar 2008. Influence of Trichoderma species on seed germination in okra. Mycopath. 6: 47-50. [ Links ]
Kamal Sharma, Ajay Kumar Mishra and Raj Shekhar Misra 2009. Morphological, Biochemical and Molecular Characterization of Trichoderma harzianum Isolates for their Efficacy as Biocontrol Agents. Journal of Phytopathology. 157: 51 -56 [ Links ]
Mausam Verma, Satinder K. Brar, R.D. Tyagi, R.Y. Surampalli, J.R. Valero 2007. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochemical Engineering Journal 37: 1-20. [ Links ]
Nashwa M.A. Sallam; K.A.M. Abo-Elyousr and M.A.E. Hassan 2008. Evaluation of Trichoderma Species as Biocontrol Agents for Damping-Off and Wilt Diseases of Phaseolus vulgaris L. and Efficacy of Suggested Formula. Egyptian Journal Phytopathology 36: 81-93 [ Links ]
Radwan M. Barakat, Fadel Al-Mahareeq, Mohammed S. Ali -Shtayeh, and Mohammad . AL- Masri 2007. Biological Control of Rhizoctonia solani by Indigenous Trichoderma spp. Isolates from Palestine. Hebron University Research Journal. 3: 1-15. [ Links ]
Saman A. 2007. Biological control oí Fusarium solani f. sp. phaseoli the causal agent of root rot of bean using Bacillus subtilis ca32 and Trichoderma harzianum RU01. Ruhuna Journal of Science 2: 82-88 [ Links ]
Skidmore, A.M. and Dickinson, C.H. 1976. Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Transactions British Mycological Society, 66: 57-64. [ Links ]
Smith, V.L., Wilcox, W.F., Harman G.E., 1991. Biological control of Phytophtora by Trichoderma. United States Patent 4996157. [ Links ]
Thung M, Rao IM, 1999. Integrated management of abiotic stresses. In: Singh SP (ed.), Common Bean Improvement in the Twenty-first Century. Dordrecht, The Netherlands, Kluwer Academic Publishers, pp, 331-370. [ Links ]
Vinale, F., D'Ambrosio, G., Abadi, K., Scala, F., Marra, R., Turra , D., Woo, S.L., Lorito, M., 2004. Application of Trichoderma harzianum (T22) and Trichoderma atroviride (PI) as plant growth promoters, and their compatibility with copper oxychloride. Journal of Zhejiang. University Science 30, 2-8. [ Links ]
Vinale, F., Marra, R., Scala, F., Ghisalberti, E. L., Lorito, M., Sivasithamparam, K. 2006. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Letters in Applied Microbiology 43:143-148 [ Links ]














