Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Tropical and subtropical agroecosystems
versión On-line ISSN 1870-0462
Trop. subtrop. agroecosyt vol.13 no.1 Mérida ene. 2011
Artículos de investigación
Impact of land use on the distribution and diversity of entomopathogenic nematodes in Embu and Taita Districts, Kenia
Impacto del uso del suelo sobre la distribución y diversidad de nematodos entomopatogénicos en los Distritos de Embu y Taita, Kenia
J. Fanuel Kawaka1, John W. Kimenju3, George Ayodo1, Shelmith W. Mwaniki2, John O. Muoma1, Sheila A. Okoth3* and George O. Orinda1
1 Department of Biochemistry and Biotechnology, Kenyatta University, P. O. Box 43844-00100, Nairobi, Kenya. Emails: fkjairo@hotmail.com; gayodo@hotmail.com; jmuoma@gmail.com; rudevsol@yahoo.com.
2 National Agricultural Research Laboratories, Kenya Agricultural Research Institute, P.O. Box 14733-00800, Nairobi, Kenya. Emails: smwaniki@yahoo.com
3 Department of Plant Science and Crop Protection, University Of Nairobi, P.O. Box 30197-00100, Nairobi, Kenya. * Corresponding author Emails: wkimenju@yahoo.com, dorisokoth@yahoo.com.
Submitted February 15, 2010
Accepted May 25, 2010
Revised received July 12, 2010
Abstract
Natural entomopathogenic nematodes (EPNs) are considered as potential biological control agents against soil-borne insect pests. This study was conducted to determine the impact of land use on the distribution, occurrence and diversity of entomopathogenic nematode community. Isolation of EPNs was done using the baiting technique and application of morphological identification methods revealed presence of the genus Steinernema. Land use intensification negatively affected the occurrence and recovery frequency in soils of Embu and Taita districts. The occurrence of EPNs was high in soils from coffee than maize and beans which had more nematodes than planted forest and napier grass followed by natural forest and tea respectively. PCR-RFLP of the internal transcribed spacer region on the ribosomal(r) DNA of the EPN isolates and digestion of the products by Alu I enzyme showed molecular variations among the isolates. The study has demonstrated that the frequency of occurrence and species variation of EPNs is different in various land uses.
Key words: Entomopathogenic nematodes; land use intensification; occurrence; diversity; PCR RFLP technique.
INTRODUCTION
Entomopathogenic nematodes are widely distributed in soils throughout the world (Adams et al., 2006; Hominick, 2002). The nematodes are considered good alternative to insect pest control due to their high reproductive potential coupled with mutualistic existence with vertebrates and plants in soils(Burnell and Stock, 2000; Gaugler, 2002). Application of non-native Entomopathogenic nematodes as biocontrol agents is used worldwide but their efficacy, reproductive potential, virulence and survival may be influenced by environmental factors. Introduction of nematode strains in new ecosystems may have negative effects on non-target organisms and partially or completely displace endemic EPNs (Lynch and Thomas, 2000).
In order to increase the efficacy of biological control products and reduce the environmental risks, knowledge on the impact of land use on the effectiveness of EPNs strains. Several studies have been conducted to determine the habitat preferences and distribution of EPNs intemperate areas of Europe: Austria (Hozzank et al, 2003), Belgium (Midituri et al, 1997). However, there is limited information available about EPNs in Africa (Waturu et al, 1997). The current study was therefore undertaken to establish the effects of land use and agro-ecosystem management on the distribution and diversity of entomopathogenic nematodes.
METHODOLOGY
The study was conducted in two benchmark sites namely Embu district in the highlands of Central and Taita-Taveta district in the Coastal highlands of Kenya. Embu site covers Irangi forest area and the adjacent farmlands. It is also recognized as a mega-biodiversity site and the adjacent farmlands with a Humic Nitisoils (Kimenju et al., 2005). Taita site covers Ngangao forest area and the adjacent farmlands in Wundanyi division. This is one of the 25 globally recognized biodiversity hotspots with a Humic Cambisols (Kimenju et al., 2005). Soil samples were taken from pre-determined sampling points under natural forests, planted forest, napier, coffee, tea, vegetable and maize and beans. Soil samples were augered from a depth of 5-30cm from three points making a triangular grid in each sampling point (Nyasani et al, 2008). A total of 20 sub-soils were taken and placed in polythene sheet where were mixed and then a sample was taken and placed in a polythene bag before transporting to the laboratory.
Nematode isolation: EPNs were isolated using the insect baiting technique and soil samples were incubated at room temperature for 24 hrs (Bedding and Akhurst, 1975). Ten last instars larvae of Gallería mellonella (Lepidoptera: Pyralidae) were placed on the surface of each soil sample, plastic boxes measuring 8 x 8 x 2 cm3 were covered with a lid, and incubated at 24 ±1°C and 55% relative humidity. Cadavers were recovered after three days, washed in tap water then placed on White traps to allow the emergence of infective juveniles (Us) (Raquel Campos-Herrera et al, 2007). All analyses were based on the relative occurrence and abundance of EPNs. Analysis of variance (ANOVA) was carried out on the data sets.
DNA extraction and PCR amplifications: Total genomic DNA was extracted from ten entomopathogenic nematodes each (mainly juveniles and adults) isolated from natural forest, planted forest, napier, tea, coffee, vegetables and maize and beans land uses using the DNeasy Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions(Susurluk et al,2003). DNA quantification and concentration was estimated using direct comparison with known DNA standards in per cent agarose minigel containing ethidium bromide (0.5μg/ml) buffered in IX TBE(89Mm boric acid and 0.5M EDTA) in horizontal electrophoresis apparatus. A 5μ1 DNA was mixed with loading dye (0.25% bromophenol blue and 40% w/v sucrose), and 2μ1 of each of the series of standard DNA solutions were each mixed with 0.4μ1 of the gel loading dye and loaded into individual wells of the agarose gel. Electrophoresis was carried out at 100 volts for 1 hour until the bromophenol blue had migrated approximately l-2cm. The intensity of the fluorescence of the unknown DNA was compared and estimated with that of the known standards. PCR amplifications of the extracted DNA were carried out for each strain using PuReTaq Ready-To-Go PCR beads in a standard 25 ul reaction volume. The reaction was set up by adding 0.25μ1 of the 18 S forward, 0.25μ1 of 26 S reverse (Vrain et al, 1992), 18.9μ1 of double distilled water and 5ul of the purified DNA. The amplifications were conducted as follows; 1 cycle at 94 °C for 2 minutes followed by 40 cycles at 94 °C for 30 seconds, 45 °C for 60 seconds, and 72 °C for 90 seconds. The last step was 72 °C for 5 minutes as suggested by Hominick et al, (1997). Agarose gel electrophoresis (180 V for 35 min) was carried out at the end of PCR amplifications using standard protocols (Reid et al, 1998). Alu I enzyme obtained from Amersham Biosciences, Freiburg, Germany was used for the digestion of the PCR products of the ITS region. The amplified rDNA products and restriction patterns were separated by electrophoresis in a two per cent agarose gel containing ethidium bromide (0.2%) in 0.5xTBE (54.0 g Tris base + 27.5 g boric acid + 20 ml 0.5 M EDTA) at 100 volt for 1.5 h
RESULTS
Morphological identification revealed that the dominant species in the soils were Steinemema spp. In Embu, soils collected from the coffee and maize/beans intercropping plots contained the highest number of samples with nematodes compared to the soils from tea farms and napier grass. Samples from planted forest had higher EPNs than natural forests. Similarly, in Taita, the highest number of EPNs was recorded in soils from maize/bean, planted forest and the napier grass where significant differences (P=0.001) between the land uses in abundance was recorded.
According to the RFLP profiles generated, all the isolates generated 3 fragments except those from coffee farm with two fragments. The fragment sizes ranged between 190bp and 330bp with the largest generated by isolates from tea while the smallest fragment was from planted forest (table 3). In Taita, the largest fragment size of 490bp was generated by all the isolates while the least fragment of 150bp was generated by isolates from napier grass and coffee soils.
DISCUSSION
Results obtained from the soils from Embu and Taita districts showed that the entomopathogenic nematodes were distributed. The observation confirmed previous studies that have shown Steinernema spp to be more frequent than Heterorhabditis spp (Hominick, 2002). Disturbance of the natural habitats through felling of indigenous trees, followed by establishment of single species plantations have been noted to cause a decline of nematode abundance and species richness (Bloemers et ai, 1997). Moderate occurrence of EPNs in natural forest soils could be due to the presence of high proportion of clay, which has poor aeration and retains a lot of water thus affecting their mobility and survival. Most EPN strains used in biological control have been isolated from sandy soils (Mra'c'ek et al, 2005).
The presence of high organic matter in forest soils which helps the proliferation of other microbes which may be antagonistic to EPNs (Nyasani et al, 2008). However, exceptions have been reported on the effect of clay soil content increasing the population, efficacy and survival of EPNs (Shapiro et al, 2000). Other biotic and abiotic soil factors have also been reported to influence distribution and occurrence of EPNs (Bednarek, 1998). Natural forests, planted forests and napier grass have large insect diversity that is hardly controlled by natural enemies than agricultural areas (Kimenjue/a/., 2005).
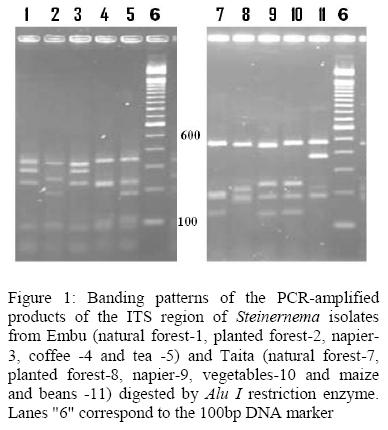
The presence of single vegetation in napier grass over many seasons is usually characterized with fewer pests and this could be responsible for reduced ability to sustain the EPNs (Mwaniki et al., 2008). The high occurrence of EPNs in coffee farms could be attributed to minimum soil disturbance in terms of tillage and low use of inorganic fertilizers and pesticides (Campos-Herrera et al, 2008). The dense canopy in coffee farms provides little fluctuations of temperature and moisture, which favour EPN survival (Nyasani et al., 2008). In vegetable and maize^ean intercrops, the occurrence of EPNs is affected by frequent cultivation which exposes them to desiccation and direct lethal rays from the sun (Shapiro et al., 1999). Besides, the use of pesticides, fresh manure, fungicides and chemical fertilizers may have detrimental effects on the survival and efficacy of EPNs (Lawrence et al., 2006). These intensive agricultural practices are unsuitable for the insects and therefore may interfere with host-parasite relationship (Caroli et al, 1996). In the intercrops, different crops are associated with different pests which could be used by nematodes for survival (Mwaniki et al., 2008). Generation of similar fragments in the isolates from Taita Taveta and Embu districts may suggest that there are species adapted to the same level of soil disturbance and land use. The unique bands generated by all the isolates revealed molecular variations within the genus Steinernema.
CONCLUSIONS
The study has demonstrated that various land uses affect the distribution, occurrence and diversity of EPNs. However, further surveys should focus on different agro ecological zones and seasons to ascertain these findings. There is also need to use more restriction enzymes to confirm the molecular variations observed during the study.
ACKNOWLEDGMENTS
The authors are grateful to the project on Conservation and Sustainable Management of Belowground Biodiversity (CSM-BGBD), a UNEP/GEF Project number GF/2715-02, for financial support. Kenyatta University, University of Nairobi and National Agricultural Research Laboratories are acknowledged for providing laboratory equipment and space while small scale farmers in Taita and Embu districts are thanked for providing free access into their farms. Finally, WWF-PBS Scholarship for Nature Conservation is acknowledged for providing study grants.
REFERENCES
Adams, B.J., Fodor A., Koppenhofer, H.S., Stackenbrandt E., Stock S.P., Klein, M.G. 2006. Biodiversity and systematic of nematode-bacterium entomopathogens. Biological Control 37:32-49. [ Links ]
Bedding, R. A., Akhurst R.J. 1975. A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica 21: 109-110. [ Links ]
Bednarek, A. 1998. The agricultural system, as a complex factor, affects the population of entomopathogenic nematode (Rhabditida: Steinernematidae) in the soil. IOBC Bulletin 21, 155-160. [ Links ]
Bloemers, G.F., Hodda, P.J.D., Lambshead, J.H., Wanless, F.R. 1997. The effects of forest disturbance on diversity of tropical soil nematodes. Oecologia 111: 575-582. [ Links ]
Burnell, A.M., Stock, S. P. 2000. Heterorhabditis, Steinernema and their bacterial symbionts-lethal pathogens of insect. Nematology 2:31-42. [ Links ]
Caroli, L., Glazer, I., Glaugler, R. 1996. Entomopathogenic nematode infectivity assay: comparison of penetration rate into different hosts. Biocontrol Science and Technology 6: 227-233. [ Links ]
Gaugler, R., Han, R., 2002. Production technology. In: Gaugler, R. (Ed.), Entomopathhogenic Nematology. CABI Publishing, Wallinford, UK, pp. 289-310. [ Links ]
Hominick W. M., 2002. Biogeography. In: R. Gaugler (ed.), Entomopathogenic nematology. CABI Publishing, Oxon, New York, pp. 115-144. [ Links ]
Hominick, W.M., Briscoe, B.R., del Pino, F.G., Heng, J.A, Hunt, D.J., Kozodoy, E., Mracek, Z., Nguyen, K.B., Reid, A.P., Spiridonov, S., Stock, P., Sturhan, D., Waturu, C, Yoshida, M. 1997. Biosystematics of entomopathogenic nematodes: current status, Protocols. [ Links ]
Hozzank, A., Wegensteiner, R., Waitzbauer, W., Burnell, A., Mra'c'ek, Z., Zimmermann, G., 2003. Investigations on the occurrence of entomopathogenic fungi and entomoparasitic nematodes in soils from lower Austria. Bull. OILB/SROP 26, 77-80. [ Links ]
Kimenju, J.W., Karanja, NK., Mutua, G.K., Rimberia, B.M.,Nyongesa, M.W. 2005. Impact of land use changes on nematode diversity and abundance. Proceedings African Crop Science Conference. [ Links ]
Lawrence, J.L., Hoy, C.W., Grewal, P.S., 2006. Spatial and temporal distribution of endemic entomopathogenic nematodes in a heterogeneous vegetable production landscape. Biological Control 37: 247-255. [ Links ]
Lynch, L.D., Thomas, M.B. 2000. Non target effects in the biocontrol of insects with insects, nematodes and microbial agents: the evidence. Biocontrol News Inform. 21: 117—130. [ Links ]
Midituri, J.S., Waeyenberge, L., Moens, M. 1997. Natural distribution of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) in Belgian soils. Russian Journal of Nematology. 5:55-65. [ Links ]
Mra' c'k, Z., Bec'va' f, S., Kindlmann, P., Jersa' kova, J., 2005. Habitat preference for entomopathogenic nematodes, their insect hosts and new faunistic records for the Czech Republic. Biological Control 34: 27-37. [ Links ]
Mwaniki, S. W., Nderitu, J. H., Olubayo, F., Kimenju, J. W. Nguyen, K. 2008. Factors influencing the occurrence of entomopathogenic nematodes in the Central Rift Valley Region of Kenya. African Journal of Ecology. 46 (Suppl. 1), 79-84. [ Links ]
Nyasani J.O, Kimenju J.W., Olubayo, F.M., Shibairo, S.I., Mutua, G.K. 2008. Occurrence of entomopathogenic nematodes and their potential in the management of diamondback Moth in Kale. Asian Journal of plant sciences 7:314-318. [ Links ]
Raquel, C.H., Escuer, M., Labrador, S., Robertson, L., Barrios, L., Gutiérrez, C. 2007: Distribution of the entomopathogenic nematodes from La Rioja (Northern Spain). J. Invertebr. Pathol, 95: 125-139. [ Links ]
Reid, A., Hominick, W., Briscoe, B. 1998. Molecular taxonomy and phytogeny of entomopathogenic nematode species (Rhabditida: Steinernematidae) by RFLP analysis of the ITS region of the ribosomal DNA repeat unit. Systematic Parasitology 37: 187-193. [ Links ]
Shapiro, D.I., Obrycki, J.J., Lewis, L.C., Abbas, M. 1999. The effects of fertilizers on black cutworm, Agrotis ipsilon,(Lepidoptera: noctuidae) suppression by steinnernama carpocapsae. Journal Nematology. 31(4S): 690-693. [ Links ]
Shapiro,D.L, McCoy,C.W.,Fares,A Obreza,T., Dou, H.2000. Effects of soil type on virulence and persistence of entomopathogenic nematodes in relation to control of Diaprepes abbreviates Environmental Entomology. 29: 1083-1087. [ Links ]
Susurluk, A., Hollmer, S., Mehta, U.K., Han, R., Tarasco, E., Triggiani, O. 2003. Molecular identification of entomopathogenic nematodes from Turkey, India, China, Italy, Norway, Albania and Germany by PCR-RFLP. 9th European Meeting of the IOBC/WPRS Working Group (Schloss Salzau, Germany), p. 101. [ Links ]
Vrain, T. C, Wakarchuk, D. A., Levesque, A.C., Hamilton, R.I., 1992. Intraspecific rDNA restriction fragment length polymorphims in the Xiphinema anericanun group. Fundamentals of Applied Nematological. 15: 563-574. [ Links ]
Waturu, C.N., Hunt, D.J., Reid, A.P. 1997. Steinernema karii spp (Nematoda: Steinernematidae), a new entomopathogenic nematode from Kenya. International Journal of Nematology, 7:68-75. [ Links ]














