Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Tropical and subtropical agroecosystems
versión On-line ISSN 1870-0462
Trop. subtrop. agroecosyt vol.13 no.1 Mérida ene. 2011
Artículos de investigación
Effect of soil fertility management practices on nematode destroying fungi in Taita, Kenya
Efecto de prácticas de manejo de la fertilidad del suelo sobre hongos nematófagos en Taita, Kenia
P. M. Wachira*1 S. Okoth1, J. Kimenju1 R.K. Mibey2 and J. Kiarie1
1 University of Nairobi P.O. Box 30197 00100 Nairobi, Kenya * Corresponding author * Email: pwachira@uonbi.ac.ke
2 Moi University P. O. Box 3900 -30100 Eldoret, Kenya
Submitted April 11, 2010
Accepted May 25, 2010
Revised received June 8, 2010
Abstract
The study aimed at identifying soil fertility practices that promoted nematode destroying fungi in the soil and the treatments comprised of Mavuno fertilizer, Triple super- phosphate and calcium ammonium nitrate (TSP+CAN), cow manure and a control where no amendments were applied. This experiment was replicated in ten farms for three planting seasons.
There were significant difference (P= 1.705 x 10-06) in occurrence of the nematode destroying fungi between soil fertility treatments. The highest mean occurrence of nematode destroying fungi was 1.6 which was recorded in soils amended with cow manure and the least was in soils from the control plots. A mean of 0.78 was recorded in soils from both TSP+CAN and Mavuno fertilizers. Plots amended with cow manure gave the highest diversity of nematodes followed by the control, then TSP+CAN and least in Mavuno with shannon indices of 0.34, 0.15, 0.13 and 0.11 respectively. Sixty percent of all the isolated nematode destroying fungi genera were from plots treated with cow manure and only twenty percent were from plots amended with the inorganic fertilizer.
Key words: Nematode destroying fungi; Arthrobotrys oligospora; organic amendments; plant parasitic nematodes.
INTRODUCTION
Taita district is a horticulture production zone where farmers experience high losses caused by the plant-parasitic nematodes (Mutsotso et al, 2005). This has led to increased cost of production through purchase of nematicides (Taita District Development Strategies 2002-2006). A survey conducted in the area revealed that farmers used inorganic fertilizers, pesticides and non-conventional methods to control nematodes and increase soil fertility (Mutsotso et al, 2005). Increasingly environmental concern on the use of nematicides have been reported (Pinkerton et al, 2000; Kerry, 2000; Larsen, 2000) which has prompted the search for alternative methods which are cost effective and environmental friendly. About 70% of fungi genera are natural enemies of plant parasitic nematodes and they have drawn much attention because of their potential as biological control agents of nematodes that are parasitic on plants and animals (Jansson and Persson, 2000; Sanyal, 2000; Birgit et al, 2002, Masoomeh, et al, 2003; Yan et al, 2005).
Although the use of inorganic fertilizers and chemical pesticides have led to considerable increases in overall food production worldwide, they have disregard the potential benefits of soil biological activities in maintaining soil health. Furthermore, overuse of these chemicals led to soil and environmental degradation (i.e., depletion or loss of soil fertility and its physical and biological components, contamination of surface and ground water) and declines in productivity in certain areas of the world (Vandermeer et al 1998). In addition, the vast majority of the world's farmers particularly in the developing countries have limited access to, external inputs necessary to apply the principles and practices of high external input agriculture (Vandermeer et al, 1998). Therefore, soil organic matter was thought to be a key factor in plant production, since it represents the main source of mineral nitrogen assimilable by crops (Chotte et al, 1998). There is evidence that soil biotic communities are associated with the aboveground vegetation (Lavelle, 2000). The high occurrence of plant parasitic nematodes in sin intensively cultivated soils of Taita Taveta could be associated with land management practices which alter soil conditions and this justifies the need to study the belowground biodiversity of these oils found at the study site (Ramakrishnan et al, 2005; Saha, 2009). The aim of the study was to identify the soil fertility management which favored buildup of nematode destroying fungi in the soil. The aim was to explore potential of NDF for use as biological control agents of plant parasitic nematodes.
MATERIALS AND METHODS
The farms were located in both Werugha and Wumingu locations of Wundanyi division, Taita district. They were divided into four plots measuring 3 x 3 meters and separated by one meter path around each plot. In one of the plots, nine kg of cow manure were broadcasted all over the plot (10 tons per ha), on the next, 0.8 kg per plot of triple super phosphate (TSP) and 0.5 kg per plot of calcium ammonium nitrate (CAN) and 0.9 kg per plot Mavuno fertilizer (blend of fertilizers containing 11 nutrients) to a third plot and these were spread uniformly over the plots. A control plot where not amendments were applied was maintained. This experiment was replicated ten times.
The plots were then planted with maize, Hybrid (H513) at a spacing of 90 x 30 cm, two seeds per hole and beans (Mwezi moja) at spacing of 30 cm in alternate rows. Soil sample was collected from each corner of the plot, then at half length of each side and at the middle of the plot. This gave a total of nine sub samples. The soils were collected at two levels, 0-10 cm and 10 - 20 cm. The two composites samples from the nine sub samples of soil collected from 0 - 10 cm and from 10 - 20 cm were used to estimate nematode destroying fungi during three consecutive seasons. The soils were collected after maturity of maize (during harvest period of maize). The soils were transported to the laboratory and kept in a cold room at about 10°C before isolation of the nematode destroying fungi.
Isolation of the fungi was done using the soil sprinkle technique described by Jaffee et ai, (1996). Tap water agar was prepared by dissolving 20 grams of agar in one liter of tap water. The medium was autoclaved and cooled to 45° C before amending it with 0.1 g/L of streptomycin sulfate to suppress bacterial growth. Approximately one gram of soil from each sampling point was sprinkled onto the surface of water agar in Petri dishes. A pure culture of plant parasitic nematodes (Meloidogyne spp.) was added into the Petri dish as baits. The plates were incubated at room temperature and observed daily under a microscope at low magnification, from the third week up to the 6th week. The examinations were focused on trapped nematodes, trapping organs and conidia of the nematode destroying fungi that grew from the soil. After the sixth week, all the fungal colonies that had emerged were sub-cultured on potato dextrose agar (PDA) to obtain pure cultures. Identification was carried as according to the key described by Cook and Godfrey, 1964.
Data analysis
Occurrence and diversity of nematode destroying fungi was compared using Frequency of occurrence, evenness, Renyi profiles and the Shannon diversity index (Kindt & Coe 2005). Principal Component Analysis and Multivariate analysis using ADE4 software were done on the temporal association of nematode-trapping fungi and soil fertility treatments (Thioulouse et al, 1997).
RESULTS
Soil fertility management practices caused significant (P= 1.705 x 10-06) difference on the occurrence of nematode destroying fungi (NDF) across the treatments. The highest occurrence of NDF was in plots treated with cow manure. Although the farmer practice (TSP + CAN) and Mavuno fertilizer were significantly different, TSP + CAN had a higher occurrence of NDF than Mavuno, while the control had the lowest (Fig. 1).
Plots treated with cow manure had the highest diversity of the fungi followed by control plots and Mavuno the lowest (Table 1).
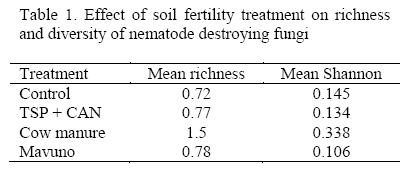
Although there were no significant differences occurrence of NDF in the two soil depths, (0-10 and 10 - 20), the 0-10 centimeters level had higher mean compared to level 10 - 20 centimeters with 1.02 and 0.9 being recorded in level 0-10 and 10-20 respectively. There was no significant difference on seasonal variation of NDF. Some NDF were not affected by the interventions (Table 2).
The nematode trapping fungi were more sensitive to soil fertility improvement interventions. Except for Acrostalamus ganoides all the endo parasitic nematode destroying fungi were not affected by the fertility management. Addition of cow manure in the soil increased the chances of isolating NDF and their diversity in the soils are presented in Fig.2.
Addition of cow manure in the soilaccounted for 61.98 of the diversity of nematode destroying fungi in the soil (Fig.3). It was observed that 60 % of the isolated genera were present in soils amended with cow manure while the remaining 40 % was due to inorganic fertilizer and control in equal proportions which accounted for 28.73% of the NDFs. Monacrosporium cionopagum and Arthrobotrys longispora species were more responsive to application of inorganic fertilizers while Arthrobotrys superba and Nematoctonous georgenous were prevalent where no chemical fertilizer had been applied. Mavuno inorganic fertilizer supported proliferation of NDFs compared to the TSP+CAN (Fig. 3).
A total of 218 isolates were identified with 58 % of the isolates belonging to the genus Arthrobotrys with A. oligospora being the most frequently isolatedspecies with 44% occurrence. Other species isolated were, A longispora, A. oligospora, A. dactyloides and A. superba. Distribution of other genera in the soils was as presented in Figure 4.
DISCUSSIONS
Nematode destroying fungi occurred in all farms however, their diversity varied with depth of soil, soil fertility management regime and the season. Gray and Bailey (1985) did not find any significant differences in the vertical distribution of the nematophagous fungi at 0-35 cm depths. However, they reported that the majority of the fungi were found in the upper organic level (0- 10). This could be explained by the fact that this is the zone with high organic matter contents which favor active microbial community with greater biomass of organisms including the nematodes which are their source of nutrition. A. oligospora, a ring former was frequent in this layer than in the second layer. This fungi was also reported to have been enhanced through addition of organic amendments in agricultural soil (Jaffee, 2004). The presence of nematodes and plants roots on the surface soil may have also caused this observation. Nematodes are usually attracted to the nematode destroying fungi and studies on cell biology on the interactions between roots cells and nematophagous fungi have indicated that the fungi have endophytic behavior in root cells (Lopez-Llorca et al, 2002). In the lower horizons, the nematode destroying fungi survive as saprophytes enabling them to compete effectively with the other organisms for available organic matter, moisture and soil nutrients.
The number of nematode destroying fungi increased each season in the plots treated with cow manure which could be explained by the fact that there was a buildup of the organic materials in the soil coupled by residual effect. In other works, highly decomposed manure had a high preference of both the nematodes and nematode destroying fungi (Mahoney and Strongman 1994).
Addition of organic or inorganic amendments to the soil cause differences in occurrence, richness, diversity and evenness of nematode destroying fungi (Wang et al, 2003). These different additions to the soil exert positive or negative impacts on the microorganisms in the soil (Sanchez 1997; Akhtar and Malik 2000). Additions of organic amendments in the soil have also been shown to stimulate the resident nematode destroying fungi (Wachira et al, 2009). In the context of agrarian practices in organic agriculture, use of organic amendments is considered a way to restore biodiversity in the edaphic environment (Garcia et al, 2004). Jaffee et al, 1998, demonstrated that organically managed plots had slightly higher number of nematode destroying fungi than the convectional plots which were treated with inorganic fertilizers, which was also confirmed in this study.
CONCLUSION
It is evident that integrated soil fertility management practices have an impact on nematode destroying fungi. Use of animal manures could restore and maintain effective populations of natural plant parasitic nematode regulatory processes in the soil.
ACKNOWLEDGMENTS
The authors acknowledge facilitation support from the Conservation and Sustainable Management of Belowground Biodiversity (CSM - BGBD) Project number GF/2715-02, a project executed by TSBF/CIAT with co financing from the Global Environmental Facility (GEF) and implementation support from the United Nations Environment Programme (UNEP).
REFERENCES
Akhtar, A., and A. Malik, 2000. Roles of organic soil amendments and soil organisms in the biological control of plant parasitic nematodes: a review. Bioresource Technology 74: 35 - 47. [ Links ]
Beentje H. I, 1988. An ecological and floristic study of the forests of the Taita Hill, Kenya. Utafiti 1:23-66. [ Links ]
Bongers, T., Bongers, M, 1998. Functional diversity of nematodes. Applied Soil Ecology 10: 239-251. [ Links ]
Bullock J. M. Pywel R.F., Mike L., Burke J.W. and Walker K. J. 2002. Restoration of biodiversity enhances agricultural production. Ecology Letters 4: 185-189 [ Links ]
Bytebier, B., 2001. Taita Hills Biodiversity Project Report. National Museums of Kenya, Nairobi. Pg 121. [ Links ]
Cheng Hu and Zhi-Ping Cao, 2008. Nematode community structure under compost and chemical fertilizer management practice, in the North China plains. Experimental Agriculture 44: 485-496. [ Links ]
Cooke, R.C., Godfrey BES, 1964. A key to the nematode-destroying fungi. Transactions of the British Mycological Society 47: 61-74. [ Links ]
Davet Pierre and Rouxel Francis, 2000. Detection and Isolation of Soil Fungi. Science Publishers, Enfield New Hampshire, USA. 188 pp. [ Links ]
Dufour, R., Guerena, M., and Earles, R. 2003. Alternative Nematode Control. NCAT Agricultural Specialists. Pest management Technical Note. [ Links ]
Elshafie, A.E., Al-Mueini, R., Al- Bahry, Akindi, A., Mohmoud, I., Al- Rawahi, S., 2006. Diversity and trapping efficiency of nematophagous fungi from Oman. Phytopathologia Mediterránea. 45: 266 - 270. [ Links ]
García-Alvarez A., Arias M., M. A. Diez-Rojo and A. Bello, 2004. Effect of agricultural management on soil nematode trophic structure in a Mediterranean cereal system. Applied Soil Ecology. 27: 197-210. [ Links ]
Githiru, M., and Lens, L., 2007. Application of fragmentation research to conservation planning for multiple stakeholders: An example from the Taita Hills, southeast Kenya. Biological Conservation 134: 271-278 [ Links ]
Gray, N.F., and Bailey, F., 1985. Ecology of namtophagous fungi: Vertical distribution in an deciduous woodland. Plant and Soil. 86: 217-223. [ Links ]
Hooper, D.J., J. Hallmann, and S.A. Subbotin, 2005. Methods for extraction, processing and detection of plant and soil nematodes. In plant parasitic nematodes in subtropical and tropical agriculture, 2nd ed. Edited by M. Luc, R.A. Sikora and J. Bridge. CAB International, pp: 53-86. [ Links ]
Jaffee, B.A., 2004. Do organic amendments enhance the nematode trapping fungi Dactylellina haptotyla and Arthrobotrys oligospora? Journal of Nematology.36: 267 - 275. [ Links ]
Jaffee, B.A., Ferris, H., and Scow, K.M., 1998. Nmetaode trapping fungi in organic and convevtional cropping systems. Phytopathology. 88: 344 - 350. [ Links ]
Jansson, H., B, Persson, C, Odeslus, R., 2000. Growth and capture activities of nematode destroying fungi in soil visualized by low temperature scanning electron microscopy. Mycologia 92: 10-15. [ Links ]
Kindt, R., Coe, R., 2005. Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. Nairobi: World Agro-forestry Center (ICRAF). Pg 196. [ Links ]
Lavelle, P., 2000. Ecological challenges for soil science. Soil Science. 165: 73-86. [ Links ]
Lopez-Llorca L.V., Bordallo, J.J., Salinas, J.,Monfort, M. L., 2002. Use of light and scanning electron micorscopy to examine colonisation of barley rhizosphere by the nematophagous fungus Vaticillium chlamydosporium. Micron. 33:61-67. [ Links ]
Mahoney, C. J., and Strongman, D. B., 1994. Nematophagous fungi from the cattle manure in four states of decomposition at three sites in Sova Scotia, Canada. Mycologia, 86: 371 - 375. [ Links ]
Mai, W.F., Mullin, P.G., 1996. Plant-parasitic Nematodes -A Pictorial Key to Genera, 5th Edition. Cornell University. Press, Ithaca, NY, USA. [ Links ]
Mutsotso B. M. Muya E. and Chirchir J. 2005. The socio-economic aspects of sustainable conservation and management of below-ground biodiversity (CSM-BGBD) in Embu and Taita bench-mark sites, Kenya. Paper prepared and presented at the global conference on conservation and management of Below Ground biodiversity in Manaus, Brazil, April 11-17,2005. [ Links ]
Muya, E.M. Muya, Gicheru, P.T. and Kariuki, C.N. 2005. Assessment of land degradation and its impacts on land use sustainability in Taita catchment. Publication, Kenya Soil Survey. Miscellaneous paper M68. [ Links ]
Ortizl G. B., Chaboussoul A., Spoerry S. and Dossol M., 2007. An Integrative Approach of the Geography of Soil Organic Matter (SOM) Management Practices to Prospect Future Below-Ground Biodiversity Erosion (BGBD), in the Taita Hills, South-East Kenya. Conference on International Agricultural Research for Development, University of Gottingen, October 9-11, 2007. [ Links ]
Pellikka P., Yihaisi, I, Clark, B., 2004. Taita Hill and Kenya, 2004 - seminar reports and journal of field excursion to Kenya Expedition reports of the Departments of Geography, University of Helsinki 40: 108-113. [ Links ]
Ramakrishnan, P.S., Saxena, K.G., Swift, M.J., Rao, K.S., and Maikhuri, R. K., 2005. Soil biodiversity, Ecological Processes and landscape management. Oxfrod and IBH Publishing Company, New Delhi. 302 pages. [ Links ]
Republic of Kenya, Taita Taveta District Development Plan, 2002-2006. Government Printer. Nairobi Kenya. [ Links ]
Saha Supradip, 2009. Sociology, Organic Fanning, Climate Change and Soil Science. Springer Netherland 3: 275-301. [ Links ]
Sanchez, P.A., 1997. Changing tropical soil fertility paradigms: from Brazil to Africa and back. In: A.C. Moniz (ed.), Plant-soil interactions at low pH. Brazilian Soil Science Society, Lavras, Brazil, pp. 19-28. [ Links ]
Spoerry Sylvie, 2006. The Taita hills and their surrounding lowlands: acquisition of a mutual dependence. MSc thesis, Montepellier, France: CNEARC, 134p. [ Links ]
Vandermeer, J., M. van Noordwijk, J.M. Anderson, C. Ong and I. Perfecto, 1998. Global change and multi-species agro ecosystems: Concepts and issues. Agriculture Ecosystems Environment 67: 1-22. [ Links ]
Wachira, P. M., Kimenju, J.W., Okoth. S.A, and Mibey, R. K., 2009. Stimulation of nematode -destroying fungi by organic amendments applied in management of plant parasitic nematode. Asian Journal Plant Sciences. 153 -159. [ Links ]
Wang, K. H., R. McSorley, R. N. Gallaher. 2003. Effect of Crotalaria júncea amendment on squash infected with Meloidogyne incognita. Journal of Nematology 36: 290-296. [ Links ]
Wang, K-H., Mcsorley, R., Marshall, A. and Gallaher, R. N, 2006. Influence of organic Crotalaria júncea hay and ammonium nitrate fertilizers on soil nematode communities. Applied Soil Ecology 31:186-198. [ Links ]
Yeates, G.W., and Bongers, T., 1999. Nematode diversity in agroecosystems. Agriculture, Ecosystems and Environment 74: 113-135. [ Links ]














