Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Tropical and subtropical agroecosystems
versión On-line ISSN 1870-0462
Trop. subtrop. agroecosyt vol.14 no.3 Mérida sep./dic. 2011
Nota corta
Biological activity and phytochemical screening of the oleoresin of Shorea robusta Gaertn. f.
Actividad biológica y exploración fitoquímica de la oleoresina de Shorea robusta Gaertn. f.
K. Sri Rama Murthy1*, N. Lakshmi1 and D. Raghu Ramulu2
1 School of Conservation Biology and Plant Biotechnology, Department of Biotechnology, Montessori Mahila Kalasala, Vijayawada - 520 010, Andhra Pradesh, India. E-mail: drksrmurthy@yahoo.com
2 Department of Botany, Sri Krishnadevaraya University, Anantapur - 515 003, Andhra Pradesh, India
* Corresponding author
Submitted January 30, 2010
Accepted July 07, 2010
Revised received June 22, 2011
Abstract
Shorea robusta Gaertn.f. oleoresin (gum) extracts are used against the skin allergies, diarrhea, dysentery, astringency and is wide spread in different parts of Eastern Ghats of Southern Peninsular India. The objective of the present study was to evaluate the antimicrobial and phytochemical activity of resin extract against pathogenic microorganisms. Successive petroleum ether, methanol, benzene and aqueous extracts of Shorea robusta resin were tested for their phytochemical constituents, antibacterial and antifungal activity. The aqueous and methanolic extracts were found to be most effective against most of the tested organisms. The results confirmed the potential of this plant in the indigenous systems of medicine.
Key words: Shorea robusta; phytochemical screening; biological activity.
Resumen
Los extractos de oleoresina (goma) de Shorea robusta Gaertn.f son empleados para el tratamiento de alergias cutáneas, diarrea, disentería y como astrigente y se encuentra presente en diferentes áreas de los Ghats occidentales de la región sur de la India. El objetivo del presente estudio fue evaluar la actividad antimicrobial y actividad fitoquímica del extracto de la resina. Se realizaron extracciones de éter de petróleo, metanol, benzeno y acuoso y se evaluaron sus constituyentes fitoquímicos y la actividad antibacterial y antifúngica. Los extractos acuosos y metanolicos fueron los más efectivos en los organismos estudiados. Los resultados confirman el potencial de esta planta en la medicina tradicional.
Palabras clave: Shorea robusta; exploración fitoquímica; actividad biológica.
INTRODUCTION
Shorea robusta Gaertn.f. (Dipterocarpaceae) is widely distributed in India, Nepal and Bhutan. In India, the species is distributed from Himachal Pradesh to Assam, Tripura, West Bengal, Bihar and Orissa, Eastern districts of Madhya Pradesh extending further to the Eastern Ghats of Andhra Pradesh. The oleoresin (gum) of the aerial parts has been reported in indigenous system of medicine as it also used as an ingredient of ointments to heal wounds, burns, pains, skin diseases and to control diarrhea and dysentery (Saraswathy et al., 1992; Pullaiah and Rani 1999; Upadhyay et al., 1998; Misra and Ahmad 1997). The literature survey reveled that no biological activity and phytochemical works has been done so far with the oleoresin of this plant. The biological activity was screened against the micro organisms causing skin allergies, diarrhea and dysentery.
Medicinal Plants have been used for centuries as remedies for human diseases, because, they contain components of therapeutic value (Nostro et al., 2000). It is estimated that there are about 250,000 species of higher plants and the majority of them have not been examined for their pharmacological activities. The antimicrobial properties of certain Indian medicinal plants were reported based on folklore information (Ram et al., 2000; Nagalakshmi et al., 2001; Jayasinghea, 2002; Chowdhury et al., 2003; Mishra et al., 2005; Lavanya et al., 2006; Murthy and Kandimalla, 2008) and a few attempts were made on inhibitory activity against certain pathogenic bacteria and fungi (Taylor et al., 1995). The plant is well reputed in traditional system of medicine and used by tribal people to treat various diseases like skin allergies, diarrhea, dysentery and also used as an astringent (Pullaiah and Rani, 1999). These are common in most of the tribal inhabitants due to lack of sanitation, potable water and awareness of hygienic food habits. Thus there is an increased need for the development of alternative antipathogenic substances. One possible approach is to screen local medicinal plants in search of suitable chemotherapeutic antibacterial and antifungal substances.
MATERIALS AND METHODS
Plant material
Oleoresin of Shorea robusta (gum) collected from the evergreen forest of Donubai hills ranges of Srikakulam district of Eastern Ghats, in February 2006. The ethanobotanical information regarding the drug-yielding plant was recorded using the standard methods (Croom, 1983, Hamilton, 1995). The information on name, part used, purpose, mode of administrations were recorded in the field notebooks as well as on the audiotapes. The sample specimens were collected in bulk quantities for analysis. Based on the folk evidences regarding the effective utilization for different skin allergies, diarrhea, dysentery and astringent, the samples were collected and screened for antimicrobial properties. The voucher specimens (No.2095) were identified with the help of regional floras (Pullaiah and Rani, 1999) and deposited in MMK Herbarium, Vijayawada.
Preparation of extracts
The plant oleoresin (gum) was shade dried (about 60g) and successively extracted with different solvents (250 ml) using a soxhlet apparatus for 6 h. The extracts were filtered and concentrated under reduced pressure, below 40°C to dryness. Phytochemical analysis for major phytoconstituents of the plant extracts were undertaken using standard qualitative methods as described by various authors (Rizk and Bashir 1980, Fadeyi et al., 1989, Odebiyi and Sofowora, 1990). The plant extracts were screened for the presence of biologically active compounds like alkaloids, flavonoids, glycosides, phenolics, saponins, steroids, tannins, Triterpenoids.
Microorganisms and culture media
Bacillus subtilis, Bacillus licheniformis, Bacillus coagulans, Bacillus cereus, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus griseus, (gram positive) Escherichia coli, Proteus vulgaris, Pseudomonas fluorescence, (gram negative) Aspergillus flavus, Aspergillus niger, Candida albicans, Penicillium chrysogenum (fungi) were obtained from the Institute of Microbial Technology (IMTECH), Chandigarh, India. The tested organisms were sub-cultured at 37°C and maintained on nutrient agar media for bacteria and sabouraud agar medium for Candida albicans.
Antimicrobial assay
The antimicrobial activity of the ethanol extracts of each sample was evaluated by using disc diffusion method (Bauer et al., 1966). Petriplates containing the aliquotes 20 ml of respective medium was seeded with selected microbial strains. Five millimeters of nutrient broth was inoculated with a loop (6 mm) of bacteria / yeast and incubated at 35°C for 6 h. One milimeter of broth was taken at 0.6 optical density (at log phase, ca.108 cells/ml) and inoculated on the nutrient agar and transferred to 180 x 20 mm petri dishes. The sterile Whatmann No. 1 filter paper discs of 6 mm diameter were impregnated with 1000-5000 ug of concentrated plant extracts and placed on the surface of the freshly inoculated medium. Standard antibiotic discs viz. Ampicillin, Tetracycline, Gentamycine, and Clotrimazole (Hi-Media, Mumbai) were used as positive controls. Ethanol and water alone served as negative controls. The assessment of antimicrobial activity was based on measurement of inhibition zones formed around the discs. The media were incubated for 24 hrs at 37C and the diameters of the inhibition zones were recorded. Three independent trials were conducted for each concentration.
RESULTS AND DISCUSSION
The aqueous, methanol, petroleum and benzene extract of oleoresin of Shorea robusta were tested (Table 1). Different extracts inhibited the growth of 12, 11,9 and 6 of the 15 used microorganisms, respectively. Aqueous extracts of Shorea robusta exhibits significant activity against Bacillus coagulans, Escherichia coli, Bacillus cereus and moderate inhibition on Salmonella typhi and Bacillus subtilis and less activity against Proteus vulgaris and Pseudomonas fluorescence. However, ethanolic extracts also exhibited significant activity against Staphylococcus aureus, S. epidermidis and Escherichia coli, moderate inhibition on Candida albicans and Bacillus coagulans. In a nutshell, methanol extract showed more significant activity. The petroleum ether and benzene extracts showed less inhibitory activity when compare with the above two extracts. The Petroleum ether showed activity against Escherichia coli, Aspergillus flavus and Candida albicans and whereas benzene extracts worked against Bacillus licheniformis, Bacillus cereus ana Aspergillus flavus.
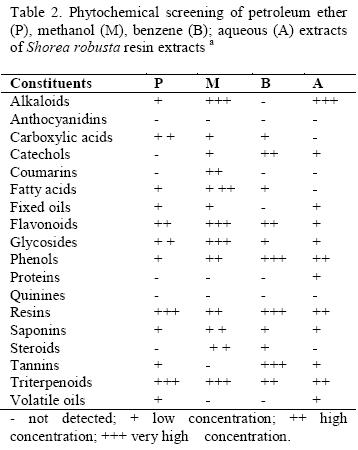
Phytochemical screening of these extracts showed the presence of alkaloids, carboxylic acids, fatty acids, phenols, saponins and steroids (Table 2.) Catechols, coumarins, proteins, tannins, volatile oils were observed in low concentrations. We suspect that the tannins found in the phytochemical analysis of the extracts could be responsible for the antibacterial activity. Triterpenoids have astringent actions, which form the basis for their therapeutic applications (Edward et al, 1970). It was found that the plants which contain triterpenoids had antimicrobial activity (Trease and Evans, 1989).
The results of antimicrobial activity are comparable with the results of the previous researches using extracts of other species like Azadirachta indica (Ram et al., 2000), Chukrasia tabularis (Nagalakshmi et al., 2001), Toona ciliata and Amoora rohituka (Chowdhury et al., 2003), Aglaia spectabilis (Lavanya et al ., 2006), Walsura trifoliate (Murthy and Kandimalla 2008). The effectiveness may be due to the cumulative action of different compounds present in the plant parts (Bai 1990). They include alkaloids, Flavonoids, triterpenoids and other compounds of phenolic nature and are classified as active antimicrobial compounds (Rojas et al ., 1992).This study revealed that the oleoresin extracts of Shorea robusta had antimicrobial activity on certain pathogens with a broad spectrum result to standard antibiotics.
CONCLUSION
Based on the results obtained in the current study, it may be concluded that Shorea robusta resin have a stronger and broader spectrum of antimicrobial activity against a number of pathogenic microorganisms and the extracts may be used to discover bioactive natural products that may serve as basic source for the development of new drugs for therapy of skin allergies, diarrhea, dysentery and astringency. The results obtained also provide support to the uses of the plants in traditional medicine. However, the toxicological analysis of the active compounds is necessary in order to assess its tolerance in the human body when administered.
ACKNOWLEDGEMENTS
KSM is grateful to the Department of Science and Technology, New Delhi for the award of DST- SERC Young Scientist grant no SR/FT/L-16/2003.
REFERENCES
Bai D. 1990.Traditional Chinese materia; a respect and prospect. Plant Medica. 56:5002. [ Links ]
Bauer A.W, Kirby M.D.K, Sherris J.C and Truck M. 1966. Antibiotic susceptibility testing by standard single disc diffusion method. American Journal of Clinical Pathology. 45: 493- 496. [ Links ]
Chowdhury R, Choudhury M and Mohammad H, 2003. Rashid A. Antimicrobial activity of Toona ciliata and Amoora rohituka Fitoterapia. 74: 155-158. [ Links ]
Croom E.M. 1983. Documenting and evaluating herbal remedies. Economic Botany. 37: 13-27. [ Links ]
Edward P.C, Varrd E.T, and Lynn RB. 1970. A Textbook of Pharmacognosy, Henry Kinston, London. [ Links ]
Fadeyi M.Q, Adeoye A.E and Olowokudejo J.D 1989. Epidermal and Phytochemical studies in the genus Boerhavia (Nyctanginaceae) in Nigeria. International Journal of Crude Drug Research; 29: 178-184. [ Links ]
Hamilton A. 1995. The People and Plants' initiative. In: Martin, G.J. (Ed.), Ethnobotany: A methods manual. WWF International. Chapman & Hall, London. [ Links ]
Jayasinghea U.L.B 2002. Jayasooriyaa C.P, Bandarab B.M.R, Ekanayakec S.P, Merlinid L, and Assantee G. Antimicrobial activity of some Sri Lankan Rubiaceae and Meliaceae, Fitoterapia. 73:424 - 427. [ Links ]
Lavanya T.M, Murthy K.S.R, Reddy N.S, and Rao K.R.S.S. 2006. Phytochemical and antimicrobial study of Aglaia spectabilis leaf extracts. Journal of Tropical Medicinal Plants. 7: 163-168. [ Links ]
Mishra V, Parveen N, Singhal K.C and Khana N.U. 2005. Antifilarial activity of Azadirachta indica on cattle filarial parasite Setaria cervi. Fitoterapia. 76, 54-61. [ Links ]
Misra LN, and Ahmad A. 1997. Triterpenoids from Shorea robusta resin, Phytochemistry. 45, 575-578. [ Links ]
Murthy K.S.R and Kandimalla, N. Antimicrobial spectrum and Phytochemical study of Walsura trifoliata (A.Juss.) Harms. (Meliaceae) bark extracts Journal of Pharmacology and Toxicology. 2008; 3: 267-71. [ Links ]
Nagalakshmi M.A.H, Thangaduri, D, Muralidharrao, D and Pullaiah T. 2001 Photochemical and antimicrobial study of Chukrasia tabularis leaves. Fitoterapia. 72: 62-64. [ Links ]
Nostro A, Germano MP, Amgelo VA, Marino A and Carínate Hi MA. 2000. Extraction methods and bioautography for evolution of medicinal plant antimicrobial activity. Letters in Applied Microbiology. 30, 379 -388. [ Links ]
Odebiyi A and Sofowora A.E. 1990. Phytochemical screening of Nigerian medicinal plants. Part III. Lloydia. 41: 234-246. [ Links ]
Pullaiah T and Rani S.S. 1999. Trees of Andhra Pradesh. Regency Publications, New Delhi, India. [ Links ]
Ram M.S, Ilavazhagan G, Sharma S.K, Dhanraj S.A, Suresh B, Parida M.M, Jana A.M, Kumar Devendrá and Selvamurthy W. 2000. Antimicrobial activity of a new vaginal contraceptive NIM-76 from neem oil {Azadirachta indica). Journal of Ethnopharmacology. 71: 377-382. [ Links ]
Rizk A, Bashir M. 1980. A chemical survey of sixty plants. Fitoterapia. 53: 35-44. [ Links ]
Rojas A, Hernandez, Pereda-Miranda R and Mata R. 1992. Screening for antimicrobical of crude drug extracts and pure natural products from Mexican medicinal plants. Journal of Ethnopharmacology. 35: 275-285. [ Links ]
Saraswathy A, Purushothaman KK, Patra A, Dey AK and Kundu AB. 1992. Shoreaphenol, a polyphenol from Shorea robusta. Phytochemistry. 31: 2561-2562. [ Links ]
Taylor R. S. 1995, Manandhar N.P and Towers G.H.N 1995. Screening of selected medicinal plants of Nepal for antimicrobial activities. Journal of Ethnopharmacology. 46: 153-159. [ Links ]
Trease, G.E and Evans W.C. 1989. Pharmacognosy, Balleire Tindal, London. [ Links ]
Upadhyay O.P, Kaushal Kumar and Tiwari R.K. 1998. Ethnobotanical study of skin treatment uses of medicinal plants of Bihar. Pharmaceutical Biology. 36: 167-172. [ Links ]