Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Tropical and subtropical agroecosystems
versión On-line ISSN 1870-0462
Trop. subtrop. agroecosyt vol.14 no.3 Mérida sep./dic. 2011
Artículos de investigación
Carotenoids digestion in african stargrass (Cynodon plectostachyus) determined with In Situ techniques in cattle
Digestión de carotenoides en pasto estrella (Cynodon plectostachyus) determinado con técnicas In Situ en bovinos
R.G. Cruz-Monterrosa1*; J.E. Ramírez-Bribiesca2; M.I. Guerrero-Legarreta1;O. Hernández-Mendo2
1 Programa de Doctorado en Biotecnología. Universidad Autónoma Metropolitana, Iztapalapa, México. Av San Rafael Atlixco 186, Col.Vicentina C.P.09340 Iztapalapa México D.F. Tel +052-0158044600. Email: cruzmonterrosa@hotmail.com.
2 Colegio de Postgraduados, Ganadería. Km. 36.5 Carr. México-Texcoco, MontecilloTexcoco. CP 56230.
* Corresponding Author
Submitted April 12, 2010
Accepted July 07, 2011
Revised received July 16, 2011
Abstract
Dry matter (DM) and total carotenoids disappearane in the rumen and intestinal passage of African stargrass (AS) were measured in 4 Holstein steers using rumen In situ and a mobile nylon bag technique in duodenum, respectively. A higher proportion of DM and total carotenoids (P<0.05) in the AS disappeared in the rumen during first 12 h. Correlation value between the disappearance of DM and total carotenoids in the rumen was 0.997 (P < 0.001). 53.0% of the carotenoids disappeared from the duodenal bags in the lower digestive tract when samples were not incubated in the rumen. Carotenoids disappearance in small intestine was lower in the samples incubated in rumen. These results show an availability of 70.0% in carotenoids into the total digestive tract. In conclusion, the AS has a high availability in the degradation of total carotenoids in the digestive tract of ruminants.
Key words: carotenoids, stargrass, cattle, digestion, disappearance, in situ.
Resumen
La material seca (MS) y la desaparición total de carotenoides del pasto estrella (PE) en el rumen e intestino del pasto estrella (PE) fueron medidas en 4 becerros Holstein, utilizando las técnicas In situ en rumen y la de bolsas de nylon móviles en duodeno, respectivamente. Una alta proporción de MS y carotenoides totales (P<0.05) de PE desaparecieron en rumen durante las primeras 12 h. El valor de correlación entre la desaparición de MS y los carotenoides totales en el rumen fue de 0.997 (P < 0.001). El 53.0% de los carotenoides desapareció de las bolsas duodenales introducidas en el intestino delgado cuando éstas no se incubaron en rumen, mientras que el porcentaje para la desaparición de los carotenoides fue mas baja en las muestras previamente incubadas en rumen. Los resultados muestran una disponibilidad de 70.0% de desaparición de carotenoides en todo el tracto gastrointestinal. En conclusión, PE tiene una alta disponibilidad en la degradación de los carotenoides totales en el tracto digestivo de los rumiantes.
Palabras clave: carotenoides, pasto estrella, bovinos, digestión in situ.
INTRODUCTION
Carotenoids are pigments synthesized by photosynthetic microorganisms and plants but not by animals. They are associated with the lipidie fractions. Their structural characteristic is a conjugated double bondsystem, which influences their chemical, biochemical and physical properties. Up to now, more than 600 carotenoids have been isolated from natural sources and they are responsible for the colors of many plants, fruits and animals (Rodriguez-Bernaldo and Costa, 2006). In addition, the synthesis of carotenoids are given by in isoprene units and plastids, occurs primarily in leaves. Depending on the species, the leaves contain 5 to 10 times more carotenoids versus the stems (Krinsky, 1989; Maoka, 2009). Carotenoids degradation occurs rapidly by oxidation. This happens mainly by light exposure and solar radiation. Subsequently, when animals ingest carotenoids, these are released from the food matrix during the physical and chemical processes that occur during the chewing and rumen fermentation (Noziére et al, 2006).
Carotenoids flow from the rumen to the small intestine with the presence of double bonds (Furr and Clarck 1997; Cardinault et al, 2006). After it, they are solubilized by the action of bile components, and follow the same absorptive pathways as other dietary lipids and become a part of the ruminant micelles absorbed in the intestinal villi. In sheep and goats, carotenoids are transported in plasma bounded to low-density lipoprotein (LDL), whereas in cattle, carotene is mainly transported in plasma bounded to high-density lipoprotein (HDL) (Yang et al, 1992). Their absorption involves several steps from the breakdown of the food matrix and release of carotenoids into the lumen of the gastrointestinal tract through their incorporation into lymphatic lipoproteins (Furr and Clarck, 1997). Although a wide range of research conducted in vitro and in situ, the microbial degradation of carotenoids in ruminal fluid is still uncertain. Some authors do not report any degradation (Dawson and Hemington, 1974: Cohen-Fernandez et al., 1976) and others researchers found degradation rates from 10% (Mora et al, 1999) to 55% (King et al., 1962).
In subtropical and tropical regions of different countries, cattle feeding is based on grazing of native grass or introduced/naturalized grass pastures. In some regions, 80.0% of farms have African stargrass in their pastures (Quero et al, 2007). High concentration of carotenoids imparts an undesirable yellow colour to cattle fat and this presentation affects the price and consumption of meat (Reynoso et al, 2004). It is suggested that the digestion in ruminants of the total carotenoids in forages should correspond with the digestion of the dry matter in the forages. This variation in proportion of total carotenoids disappearing from forages in the rumen could be a contributing factor in the wide variation in bio-availability of carotenoids of natural forages. Using in situ and the mobile bag technique, the disappearance from the bags of total carotenoids was measured during incubation in the rumen and passage through the lower digestive tract of steers.
MATERIAL AND METHODS
Sample Collection
African stargrass (Cynodon plectostachyus) samples were obtained in Research Centre of Agriculture and Animal Science "Las Margaritas, INIFAP". This place is located in a tropical region in northwest of Puebla state, Mexico. Grass samples were collected in rainy season from at least 7 different areas within each region and these were maintained in a cooler into black plastic bags, removed the excess air and closed the bag tightly to subdued lighting and minimize the destruction of carotenoids. Then, in semi dark room, the grass samples were separated in green and dead material. The majority of grass selected in this trial had an elongation stage of growth; maturity class, according to the methodology proposed by Moore et al. (1991). Fresh pasture forage samples were immediately chopped finely (less than 3 mm) with scissors, and all sub-samples (9 sub-samples/sample) were mixed and a composite sample was prepared from 7 sampling sites. It was frozen at -20 °C until the onset of in situ determinations. The upper portion, considered as representative of the eatable pasture, was dried and ground (1-mm Wiley mill screen). Forage DM content was determined by oven drying at 60 °C for 24h. Sample by duplicate were analyzed for OM by ashing at 600 °C for 12h, nitrogen (Kjedahl N), acid and neutral detergent fibre (NDF) according to Weizhong and Udén (1998).
Steers and Feeding
Four Holstein steers cannulated in the rumen and proximal duodenum were used in the study. Surgeries were preformed 2 months prior to the start of the experiment. The protocol for surgical preparation, and maintenance and handling of steers was approved by the Colegio de Postgraduados, Animal Care Administrative Advisory Committee. Steers were housed in a slotted-floor metabolism. The weight of steers averaged 384 ± 60 kg of BW.
Steers were fed with alfalfa hay two times daily at 0700 and 1900 h in two equal portions. DMI was measured during 5 days. Thereafter, each steer was restricted to 90.0% of its respective ad libitum DMI. 20 grams of mineral supplement (NaCl > 81.0%, Ca, 10.0%, P 5.0%, Mn, 0.2%, Fe, 0.1%, Mg, 0.1%, Cu, 0.025%, Zn 0.01% I, 0.007%) was offered daily. Alfalfa hay had a late maturity state and it contained (g kg-1 DM): Organic matter 897, Crude protein 130, Neutral detergent fiber 607 and total carotene 125 mg/kg.
In Situ Rumen Incubation of samples
Nylon bags (5x3 cm; 47-μm pore size) were filled with 1.2 g of fresh grass composite sample and were closed using a special tie with hemp cord. Each grass composite sample was incubated in six replicates per each hour (12, 24, 48, 72 h) within each steer. Nylon bags were maintained into of ruminal fluid compartment with small chain and these were then removed from the chain and were manually washed with tap water until the rinse water remained clear. Three additional bags were washed with water without rumen incubation and were considered the 0 h incubation. Subsequently, samples were taken from the rumen, were conserved in amber glass flask until to transfer in duodenal bags according to each time periods of ruminal incubation. Each incubated samples (no incubated, 0 h) were used to determinate total carotenoids analysis; and another part of the same samples were dried in an oven at 60 °C until a constant weight to determine DM disappearance and adjust the carotenoids disappearance in dry matter basis (Mora et al, 1999).
In Situ Intestinal Incubation of Samples
Nutrient disappearance during passage through the intestine was determined using the mobile nylon bag technique (De Boer et al, 1987). 1.2 g of fresh grass samples of 0 hour and previously incubated in the rumen of each steer for 12, 24, 48 and 72 h were introduced into the duodenal bags and these were successively incubated in an HC1 solution for 1 h at pH 2.4 and in an HCl-pepsin solution (lg pepsin + 0.1 N HCL/L) for 2 h at 39°C. Thereafter, the bags were introduced every 20 min into the small intestine through the duodenal cannula. After recovery from the feces, the bags were hand washed with slight pressure for a period of 5-7 min in each sample, until the water was clear. Residual DM (60 °C for 24 h) and total carotenoids (fresh sample) were conducted on the pooled residues from the mobile nylon bag.
Carotenoids Analyses
Carotenoids were extracted from fresh grass samples instead of dry samples to prevent the destruction and these were adjusted with the proportion of dry matter calculated in spare samples. Forage samples (0.500 g) were transferred to amber bottles of 50 mL and 20 mL of acetone were added to the samples. The solutions were stored at 4 °C in a refrigerator to extract all pigments. After 48 h, the solutions were filtered through filter paper (Whatman GF/A) into a 500 mL separately funnel and its contents were homogenized with 20 mL of ether petroleum. After 100 mL of distillated water were added to the samples and it were stabilized from 5 to 10 min, the top hexane layer was collected immediately; this procedure was repeated 2 or 3 times. Free extract acetone were obtained and then about 10 mL of sodium hydroxide were added, and it were shacked into a 500 mL separately funnel. After that the complete samples were washed with distillated water to eliminated sodium hydroxide 40%, this procedure were corroborated with 3 drops of phenolphthalein, the samples must to be washed to the pink color. Then, add 20 mL of Sodium sulphate (5%) and shake again into a 500 mL separately funnel and it was washed with distillated water. The top hexane layer was collected immediately and Petroleum ether was added (50 mL) and the carotenoids extracted into the layer of petroleum ether; this procedure was repeated 3 times. Absorbency was measured using a spectrophotometer at a wavelength of 454 nm. Concentrations of carotenoids were calculated by using an external standard curve and then were adjusted by percent recovery of the added internal standard. The internal standard for carotenoids was prepared with 200 mL of a 1 mL/mg solution of (3-carotenal Type 1, aprox 95% UV Sigma® C975-56, 068K2561. It was mixed with 200 mL of 0.1 M methylhydroxylamine (in 0.1 M buffer, adjusted to pH 6.7) and incubated for 24 h in a water bath at 37 °C.
Care was taken throughout the experimental and analytical procedure to protect samples from natural light (i.e., samples and test tubes were wrapped in aluminum foil to keep light out and extraction under dim artificial light) (Hoppe et al, 1972).
Calculations
The percent disappearance of DM and total carotenoids at 12, 24, 48 and 72 h incubation in the rumen were calculated from the quantity of nutrient placed in the bag and left in the residue after incubation in the rumen (ruminal disappearance). Disappearance in the intestinal tract was calculated by the difference between the rumen residue after each 0, 12, 24, 48 and 72 h of incubation and the portion remaining in samples recovered from feces.
Statistical Analyses
In situ data were analyzed using average values of six replication samples and analyzed as a randomized complete block design using the PROC GLM of SAS (2003). Differences among means were compared at each incubation time point with Tukey's multiple range test.
RESULTS
Chemical composition
The chemical composition of African stargrass (Cynodon plectostachyus) on dry matter basis was: dry matter, 88.95%; organic matter, 78.63%; crude protein, 12.0%; neutral detergent fiber, 60.73%; acid detergent fiber, 28.97% and total carotenoids 627 mg/kg.
Ruminal disappearance
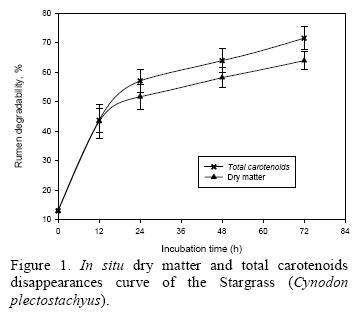
The percentage disappearances of the dry matter and total carotenoids in the African stargrass are presented in Figure 1. More than 40% of the dry matter and carotenoids concentration in the African stargrass disappeared drastically in the rumen during the first 12 h, and were stabilized from 24 to 72 h. A similar trend (P < 0.05) was seen in the disappearance of the total carotenoids in the African stargrass. Consequently, the correlation value for African stargrass between the disappearance of DM and total carotenoids in the rumen was 0.997 (P < 0.001). With the data of the dry matter and total ruminal carotenoids degradation the following models were fitted: Dry matter disappeared, % = 13.7487 + 2.9919X - 0.066X2 + 0.0005 X3 (r2 = 0.99) and Total carotenoids disappeared, % = 13.2506 - 3.227X- 0.689X2 + 0.0005X3 (r2 = 0.99).
Lower digestive tract
A 53.0% of the carotenoids in the African stargrass disappeared from the duodenal bags in the lower digestive tract when it was not incubated in the rumen. Logically, bags with longer incubation in the rumen contain less carotenoids and therefore the efficiency of disappearance in the small intestine decreased (P < 0.05, Table 1). Consequently, there was no correlation between the disappearance of DM and total carotenoids in small intestine.
Total disappearance
The extent of intestinal digestibility of carotenoids depends on incubation time of African stargrass in the rumen; longer incubation time induces higher carotenoids degradability and lower intestinal digestibility of undegraded carotenoids (P < 0.05, Table 1). The concentration of carotenoids in hay alfalfa was very low (125 mg/kg). Our results show that apparent availability in the total digestive tract was higher to 70 % of intake when the samples were incubated in the rumen.
DISCUSSION
Chemical composition
In the present study, protein and NDF concentrations in African stargrass were similar to those reported by Perez et al. (2001). Recently, Dunne et al. (2009) demonstrated that the content of carotenoids, most fresh green leafy material will contain between 200 and 700 mg carotene/kg DM.. The concentration of total carotenoids in African stargrass (627 mg/kg DM) was high or similar compared with other data reported on pastures: 350 in a pasture consisting of Dactylisglomerata, Meum athamanticum and Archillea millefollium (Calderón et al, 2006), 300 to 500 mg/kg DM in a mixture of twelve pasture crops (Visser and Blair 1991), 430 to 700 mg/kg DM in mixture of Lolium, Dactylis and Festuca grass (Prache et al, 2003), and 350 - 520 mg/kg DM of total carotenoid concentration in grass silage from 350 to 520 mg/kg DM (Chauveau-Duriot et al, 2005; Calderón et al, 2007). In tropical regions of Mexico, Reynoso et al. (2004) measured the carotenoids concentration in two grass species (Digitaria decumbes: Pangóla grass and Cynodon dactylon: Bermuda grass). The total carotenoid content was 334 mg/kg DM in humid tropic and 116 mg/kg DM in dry tropic. In the present study, carotenoid concentration was double to that found previously in the same humid tropic region, factor that may influence these differences may be caused by phenolical stage of forage, climatic conditions (Guimaraes et al, 1992) and forage species should be an important factor influencing carotenoids content in pasture.
Ruminal disappearance
As was mentioned, more than 40% of the dry matter and total carotenoids in the African stargrass disappeared in the rumen during the first 12 h. Other researchers have reported that the average ruminal digestibility of tropical forages is 53 to 63%, and has to be lower with the degree of maturation (Moore et al, 1981; Arthington and Brown, 2005). These results supported other previous findings (Mora et al, 1999) that the disappearance of carotenoids from forages in the rumen can be related to the dry matter and cellular content (Britton, 1995). In situ studies performed with bovine ruminal fluid incubated for 4 to 16 h reported a degradation of β-carotene from 0-30 to 0-20 (King et al, 1962; Keating et al, 1964). Similarity, Rode et al. (1990) quantified vitamin A disappearance in ruminal fluid from cattle fed concentrate, hay, or straw diets. These authors estimated effective ruminal degradations of vitamin A for these diets of 67, 16, and 19%, respectively; they concluded that the disappearance is attributed to microbial enzymatic activity. The reason is that vitamin A and carotenoids have four double bonds in its side chain (Simpson, 1983), these are susceptible to reduction in the anaerobic environment of the rumen because double bonds are targets for electron and proton deposition (biohydrogenation) by a number of ruminal microbes (Britton, 1995). Other in vitro and in vivo studies demonstrated that p-carotene was weak or not destroyed by rumen microorganisms (Dawson and Hemington 1974; Mora et al, 1999). The differences in the degradation of carotenoids in the rumen may be due to the characteristics of the microbial population, which are influenced by diet or grazing (Rode et al, 1990).
Lower digestive tract
There are many factors can influence the initial release of carotenoids from the forage matrix and their subsequent dissolution in lipidie drop in the stomach and duodenum. Generally, total carotenoids are present in complexes with proteins or in semicrystalline structure, and they have to be transferred or dissolved in the lipid phase before they are absorbed. This data on passage of carotenoids through the gut are particularity based on non-ruminant animals and humans. Serrano et al. (2005), showed a significant inverse correlation between small intestine availability of carotenoids (lutein + (3-carotene) and content of lignin, nonstarch polysaccharides and resistant protein in green leafy vegetables. It should directly affect the intestinal availability of carotenoids acting as a barrier to the action of digestive enzymes, and to the release of carotenoids from the food matrix.
In ruminants, this mechanism remains unknown but could likely present some specificity as a consequence of the ruminal metabolism of dietary lipids which induces a modification of the composition of the lipids entering in the duodenum and biliary secretions may also influence carotenoid composition in the digestive tract (Noziere et al, 2006). Intestinal absorption of carotenoids in animal feeds varies tremendously from as low as 0.08 to 0.6 (Yang et al, 1992; Cardinault et al 2006; Noziere et al, 2006). The results from the present study suggested that a proportion of carotenoids in pasture can escape microbial fermentation and reduction in the rumen. This suggests that the disappearance of carotenoids in the lower tract depends on the amount of carotenoids in the sample. The samples were not incubated in the rumen contained more carotenoids and the disappearance was higher in the small intestine. Also, forage samples incubated in the rumen lost more carotenoids with increasing time. The disappearance of carotenoids from samples introduced into the duodenal cannula depending on the amount of residual carotenoids in samples incubated in the rumen.
Total disappearance
Results on apparent digestibility of carotenoids have already been published. Some authors reported a low apparent digestibility of carotene (0.15) in sheep or cows given natural or synthetic sources of carotenes (Hoppe et al, 1972; Cohen Fernandez et al, 1976; Kumar et al, 1981). But other studies have shown a higher (0.55) apparent digestibility of carotene (Wing, 1969; Mora et al, 2001). No clear effects of sources or amounts of carotenoids ingested, may explain divergence in results between studies. In general, total carotenoids concentration and digestion are regulating by over maturity and degree of lignification of the grass (Dunne et al, 2009), so carotenoids are most abundant in fresh pasture and, may not be but rather by differences in analytical methodologies. Specifically, when one method is validated in fresh plant tissues must have an intra- and inter-day variation coefficient between 0-03 to 0-08 for all carotenoids and must recover between 0-75 to 1-00 (Cardinault et al, 2006).
CONCLUSION
The disappearance of carotenoids and DM from the bags in the rumen showed high degradation fates, which differ in the ruminal period incubations. However, in the total tract gastrointestinal, a large proportion of Cynodon plectostachyus can be secreted and it is highly available for absorption in the digestive tract.
REFERENCES
Arthington, J.D., Brown, W.F. 2005. Estimation of feeding value of four tropical forage species at two stages of maturity. Journal of Animal Science. 83: 1726-1731. [ Links ]
Britton, G. 1995. Structure and properties of carotenoids in relation to function. J. Fed. Amer. Soc. Experim. Bio. 9: 1551-1558. [ Links ]
Calderon, F., Tornambé, G., Martin, B., Pradel, P., Chauveaut-Duriout, B., Nouziere, P. 2006. Effects of mountain grassland maturity stageand grazing management on carotenoids in sward and cow's milk. Animal Research. 55: 533-544. [ Links ]
Calderon, F. Chauveau-Duriot, B., Pradel, P., Martin, B., Graulet, B., Doreau, M., Noziere, P. 2007. Variations in Carotenoids, Vitamins A and E, and Color in Cow's Plasma and Milk Following a Shift from Hay Diet to Diets Containing Increasing Levels of Carotenoids and Vitamin E. Journal Dairy Science. 90: 5651-5664. [ Links ]
Cardinault, N., Doreau, M., Poncet, C, Nozieeret, P. 2006. Digestion and absorption of carotenoids in sheep given fresh red clover. Animal Science. 82: 49-55. [ Links ]
Chauveau-Duriot, B., Thomas, D., Portelli, J., Doreau, M. 2005. Carotenoids content in forages variation during conservation. Rencontres Recherches Ruminant. 12: 117. [ Links ]
Cohen-Fernandez, S., Budowski, P., Ascarelli, I., Neumark, H., Bondi, A. 1976. Low utilization of carotene by sheep. Inter. Journal of Vitamin Nutrition Research. 46: 446-453. [ Links ]
Dawson, R.M., Hemington, N. 1974. Digestion of grass lipids and pigments in the sheep rumen. British Journal Nutrition. 32: 327-340. [ Links ]
De Boer, G., Murphy, J.J., Kennelly, J.J. 1987. Mobile nylon bag for estimating intestinal availability of rumen undegradable protein. Journal Dairy Science. 70: 977-982. [ Links ]
Dunne, P.G., Monahan, F.J., O'Mara, F.P., Moloney, A.P. 2009. Colour of bovine subcutaneous adipose tissue: A review of contributory factors, associations with carcass and meat quality and its potential utility in authentication of dietary history. Meat Science. 81:28-45. [ Links ]
Furr, H.C., Clark, R.M. 1997. Intestinal absorption and tissue distribution of carotenoids. Review. Nutrition Biochemical. 8: 364-377. [ Links ]
Guimaraes, A.M., Saliba, E.O.S., Rodriguez, N.M., Moreira, P.K. 1992. Seasonal variation of vitamin A, macro and microelements in grass, plasma and liver of Neloreheifers kept in Brachiaria decumbens pasture. Arquivos Brasileiros de Medicina Veterinaria e Zootecnia. 44: 57-66. [ Links ]
Hoppe. P., Tiews, J., Last, W., Klee, W., Koch, G. 1972. Plant carotenoids as an indicator of the determination of green forage digestibility in ruminants. Futtermittelkunde 30: 65-76. [ Links ]
Keating, E.K., Hale, W.H., Hubbert, F. Jr. 1964. In vitro degradation of vitamin A and carotene by rumen liquor. Journal of Animal Science. 23: 111-117. [ Links ]
King, T.B., Lohman, T.G., Smith, G.S. 1962. Evidence of rumeno-reticular losses of Vitamin A and carotene. Journal of Animal Science. 21 (Suppl.) 1002 (abstr. [ Links ]).
Krinsky, N.I. 1989. Antioxidants functions of carotenoids. Free Radical Biological Medical. 7: 617-635. [ Links ]
Kumar, M.N.A., Sundareshan, K., Sampath, S.R. 1981. Effect of feeding varying quantities of forages on vitamin A content of blood and milk of cows. Indian Journal of Animal Science. 51:21-25. [ Links ]
Maoka, T. 2009. Recent progress in structural studies of carotenoids in animals and plants. Review. Archives of Biochemical and Biophysical. 483: 191-195. [ Links ]
Mora, O., Romano, J.L, Gonzalez, E., Ruiz, F.J., Shimada, A. 1999. In vitro and in situ disappearance of beta-carotene and lutein from lucerne (Medicago sativa) hay in bovine and caprine ruminal fluids. Journal Science Food Agriculture. 79: 273-276. [ Links ]
Mora, O., Romano, J.L., González, E., Ruiz, F.J., Gómez, R., Shimada, A. 2001. Presence of fed betacarotene in digesta, excreta, blood, and hepatic and adipose tissues of Holstein steers. Canadian Journal of Animal Science. 81: 133-139. [ Links ]
Moore, J.E., Worrell, M.A., Abrams, S.M., Ocumpaugh, W.R. 1981. Quality of tropical perennial grass hays. In Florida Beef Cattle Res. Rep., Univ. of Florida, Gainesville, pp. 40-44. [ Links ]
Moore, K.J., Moser, L.E., Vogel, K.P., Waller, S.S., Johnson, B.E., Pedersen, J.F. 1991. Describing and quantifying growth stages of perennial forage grasses. Agronomy Journal. 83: 1073-1077. [ Links ]
Noziére, P., Graulet, B., Lucas, A., Martin, B. Grolier, P., Doreau, M. 2006. Carotenoids for ruminants: from forages to dairy products. Animal Feed Science and Technology. 131: 418-450. [ Links ]
Pérez, P.J., Alarcón, Z.B., Mendoza, M.G.D., Barcena, G.R., Hernández, G.A., Herrera, H.J.G. 2001. Response of kudzu as protein bank on steers grazing african stargrass. Técnica Pecuaria México. 39: 39-52. [ Links ]
Quero, C.A., Enriquez, Q,J,F,, Miranda, J.L. 2007. Forage species evaluation in tropical America, Advancement or Status Quo. Interciencia 32: 567-571. [ Links ]
Prache, S., Priolo, A., Grolier, P. 2003. Persistence of carotenoid pigments in the blood of concentrate-finished grazing sheep: its significance for the traceability of grass-feeding. Journal of Animal Science. 81: 360-367. [ Links ]
Reynoso, C.R., Mora, O., Nieves, V., Shimada, A., González de Mejía, E. 2004. P-Carotene and lutein in forage and bovine adipose tissue in two tropical regions of Mexico. Animal Feed Science and Technology. 113: 183-190. [ Links ]
Rode, L.M., McAllister, T.A., Cheng, K.J. 1990. Microbial degradation of vitamin A in rumen fluid from steers fed concentrate, hay or straw diets. Canadian Journal of Animal Science. 70: 227-233. [ Links ]
Rodriguez-Bernaldo, A., Costa, H.S. 2006. Analysis of carotenoids in vegetable and plasma samples: A review. Journal Food Compare. Annals 19: 97-111. [ Links ]
SAS. 2003. Statistical Analysis Systems User's Guide. Statistics Version 9.1. SAS Institute Inc. Cary, NC, USA. [ Links ]
Serrano, J., Go, I., Saura-Calixto, F. 2005. Determination of beta-carotene and lutein available from green leafy vegetables by an in vitro digestion and colonic fermentation method. Journal Agriculture Food Chemical. 20: 2936-2940. [ Links ]
Simpson, K.L. 1983. Relative value of carotenoids as precursors of vitamin A Proceedings Nutrition Society. 42: 7-17. [ Links ]
Visser, F.R.., Blair, K.A. 1991. B-carotene content of twelve pasture crops, measured over one dairy season. NZDRI report no. FV91R11. Palmerton North Zealand. [ Links ]
Weizhong, C, Udén, P. 1998. An alternative oven method combined with different detergent strengths in the analysis of neutral detergent fiber. Animal Feed Science and Technology. 74: 281-288. [ Links ]
Wing, J.M.A. 1969. Effect of source and season on apparent digestibility of carotene forage by cattle. Journal of Dairy Science. 52: 479-483. [ Links ]
Yang, A., Larsen, T.W., Turne, R.K. 1992. Carotenoid and retinol concentrations in serum, adipose tissue and liver and carotenoid transport in sheep, goats and cattle. Australian Journal of Agriculture Research. 43:1809-1817. [ Links ]