Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Tropical and subtropical agroecosystems
On-line version ISSN 1870-0462
Trop. subtrop. agroecosyt vol.14 n.2 Mérida May./Aug. 2011
Artículos de investigación
Integrated management of parasitic plant Striga hermonthica in maize using Fusarium oxysporum (mycoherbicide) and post-emergence herbicides in the Nigerian savanna
Manejo integrado de la planta parásita Striga hermonthica en el maíz empleado Fusarium oxysporum (micoherbicida) y herbicidas post-emergencia en la sabana de Nigeria
E.I. Magani1*, A. Ibrahim2 and R. I. Ahom1
1 Department of Crop and Environmental Protection, University of Agriculture P.M.B 2373, Makurdi, Nigeria; *Corresponding AuthorE-mail:- m.enochistifanus@yahoo.com
2 Department of Agronomy, Nasarawa State University, Keffi, Nasarawa, Nigeria.
Submitted August 30, 2010
Accepted December 01, 2010
Revised received December 10, 2010
Abstract
The efficacy of a granular mycoherbicide formulation based on Fusarium oxysporum and post-emergence herbicides for the control Striga hermonthica was evaluated. Four fungal treatments were used: F. oxysporum followed by 2, 4-D, F. oxysporum followed by supplementary hoe weeding, F. oxysporum followed by Triclopyr and a control (No F. oxysporum) in two maize varieties (Across 97 TZL and farmer's local variety). The maize variety Across 97 TZL significantly delayed the emergence of Striga as compared to the farmer's local. The highest number of maize plant infected with Striga/shoot count was recorded at Makurdi and the farmer's local variety. Similarly, in the Striga control methods, the hoe-weeded check recorded significantly more Striga/shoot count when compared to all other control treatments. Highest maize grain yields were obtained in 2009; at Makurdi; Across 97 TZL and plots that received F. oxysporum followed by post-emergence application of either Triclopyr, 2,4-D each at 0.36 kg active ingredient/ha.
Keywords: Biocontrol; Fusarium oxysporum; herbicides; Striga hermonthica; maize; mycoherbicide.
Resumen
Se evaluó la eficacia de un micoherbicida granular basado en Fusarium oxysporum y herbicidas post-emergencia para el control de Striga hermonthica. Se emplearon cuatro tramientos con hongos: F. oxysporum seguido de 2, 4-D, F. oxysporum seguido de deshierbe, F. oxysporum seguido de Triclopyr y un control (No F. oxysporum). Dos variedades de maíz fueron empleadas (Across 97 TZL y una variedad local). La variedad de maíz Across 97 TZL retrasó significantivamente la emergencia de Striga en comparación con la variedad local. El mayor número de plantas de maíz infectadas con Striga se registró en Makurdi con la variedad local. De manera similar, para los métodos de control de Striga, en el deshierbe se encontró mayor cantidad de Striga en comparaicón con los otros métodos. La mayor producción de maíz se obtuvo con Across 97 TZL y en las parcelas que recivieron F. oxysporum seguido por la aplicación post-emergencia de Triclopyr o 2,4-D a razón de 0.36 kg de ingrediente activo/ha.
Palabras clave: Biocontrol; Fusarium oxysporum; herbicidas; Striga hermonthica; maíz; micoherbicida.
INTRODUCTION
Striga hermonthica (Del.) Benth is a parasitic angiosperm of a number of economically important crops within the Poaceae family including Sorghum ( Sorghum bicolor (L.) Moench), maize (Zea mays L.), millet (Pennisetum americanum L.) and rice (Oryza sativa (L.) (Stewart, 1990; Johnson et al., 1997). Striga is very difficult to control and the use of a multiple integrated management approach for controlling Striga infestations has been commonly proposed (Carson, 1988; Parker, 1991; Thalouaran and Fer, 1993). Despite substantial research efforts, no effective means of controlling Striga have been achieved to date. Biological control is one option that has received attention recently. Numerous surveys for pathogens as possible biological control agents of Striga species demonstrate the growing interest for using alternative strategies to combat these noxious weeds (Abbasher et al., 1995; Ciotola et al., 1995; Kroschel et al., 1996; Marley et al., 1999). Most of these studies have focused on soil microorganisms of the genus Fusarium. In glasshouse experiments, F. oxysporum isolates have been found to be highly pathogenic against S. hermonthica (Kroschel et al., 1996; Abbasher et al., 1998).
Yield reduction caused by S. hermonthica can be up to 79% even under good management (Lagoke et al., 1997). One of the reasons why Striga has devastating impacts on the growth and yield of cereals relates to its dual mode of action. First, Striga plants compete effectively with host for carbon, nitrogen and other inorganic elements (Gurney et al., 1995; Frost et al., 1997). Secondly, the parasite has a so-called 'phytotoxic effect' on the host plant within days of attachment (Berner et al., 1995; Gurney et al., 1995). A very small parasite biomass, with attachments of less than 4mm in size, results in a large reduction in host height, biomass and eventual grain yield (Gurney et al., 1995).
An integrated management approach, if properly designed, using a combination of control measures has the potential to provide a lasting solution to the Striga problem (Emechebe et al., 1991). Therefore, the objective of this study was to evaluate the efficacy of a combination of granular mycoherbicide formulation of F. oxysporum and post-emergence herbicides for the control of the parasitic plant S. hermonthica in maize in the Nigerian Savanna.
MATERIALS AND METHODS
Preparation of Fusarium oxysporum
The biocontrol agent F. oxysporum was produced on gritted (whole grain broken into smaller pieces) maize grains in the laboratory as described by Marley et al (1999). Fusarium oxysporum (isolate PSM 197) was isolated from S. hermonthica stems and single spore isolates made into stock cultures. They were maintained on potato dextrose agar (PDA) amended with streptomycin (Difco®) and stored in the refrigerator at 4°C. Starter cultures were made when required. Mass production of inoculum was made on gritted maize grain. Gritted grain (500g) was placed in 1-L flat-bottomed conical flasks each containing 250ml of sterile distilled water. Flasks were shaken to ensure that the substrate was properly moistened and excess water was poured off prior to autoclaving for 1 hr at 121°C (103. 5 kPa). After cooling each flask was inoculated with three 5-mm diameter agar plugs of the isolate and then incubated at 28° C for 7 days. During the incubation period, each flask was shaken daily to allow for full colonization of the grains by the pathogen. Colonized grains were harvested 14 days after inoculation and stored in refrigerator at 4° C for use when required as the mycoherbicide.
Field evaluation
Two experiments were conducted in 2008 and 2009 cropping seasons at the Teaching and Research Farm of the University of Agriculture, Makurdi (07° 14'N, 08 37'E) and the Model Extension village, Danka-Sarki, Lafia (08° 3' N and 07° 31'E) in the Southern Guinea Savanna of Nigeria. The two sites were naturally and heavily infested with S. hermonthica.
The two experiments were established on the 28th May and 16th June in 2008 and 2009, respectively. In each year, the two experimental sites was ploughed, harrowed and ridged at 0.75m apart. Each of the two maize varieties (Across 97 TZL and a farmer's local variety) were planted 50cm apart along the ridges. At each planting ridge, 2 g (53.33 Kg/ha) of mycoherbicide in each treatment was applied pre-sowing in the planting hole. Four treatments were used as follows: F. oxysporum followed by 2, 4-D, F .oxysporum, followed by supplementary hoe weeding, F. oxysporum followed by Triclopyr and a control (No. F. oxysporum but hoe- weeded). The experiments were laid out in a split-plot design with triplicates in the two years. The two maize varieties formed the main plot treatments, while the Striga fungal control methods (F. oxysporum either followed by 2, 4-D, supplementary hoe weeding, Triclopyr at 6 weeks after sowing (WAS) and a control) formed the sub-plot treatments. The gross and net plot sizes were 9 and 4.5m2 (4 ridges and 2 rides each of 3m length), respectively. Spot application of fertilizer was carried out at 120Kg N/ha, 60Kg, P2 05/ha and 60Kg K2 0/ha to maize using 15-15-15 N-P-K compound fertilizer at 3 (WAS). The post-emergence herbicides (2, 4-D and Triclopyr) were applied at 6 weeks after sowing at 20% Striga infestation using a knapsack sprayer (CP3) with spray volume of 250L/ha and a yellow nozzle.
Observations made were: Number of days to first Striga emergence, number of maize plants infected by Striga, Striga shoots per unit area, number of capsules per Striga plant, crop vigour score, maize stand count, weight of 1000 grains and grain yield. The data collected were subjected to analysis of variance (ANOVA) and means were compared using Least Significant Difference (LSD) (α =0.05).
RESULTS AND DISCUSSION
Number of days the Striga emergence differed significantly in the two years, two locations, among the maize varieties tested and the different Striga control methods (Table 1). In 2009, there was a significant delay in the emergence of Striga (48 days after sowing, DAS) as compared to 2008 (42 DAS). At the Lafia location, there was a significant 7 day delay in the emergence of Striga days (49 DAS) as compared to Makurdi (42 DAS). This may be due to higher Striga seed bank density at the Makurdi location than that of Lafia. Maize variety Across 97 TZL significantly delayed the emergence of Striga by 10 DAS as compared to the farmer's local variety (Table 1). Among the different Striga control methods, the result indicated early emergence with the hoeweeded check. This result agrees with an earlier report that pre-planting inoculation with the pathogen caused between 7 and 14 days delay in the emergence of S. hermonthica (Marley, et al., 2005). There was a significant interaction between year and location on number of days to Striga emergence (Table 2). The 2009 X Lafia interaction delayed Striga emergence the most (58 DAS) when compared to earliest with 2009 X Makurdi and 2008 X Lafia interactions by 39 and 38 days, respectively.
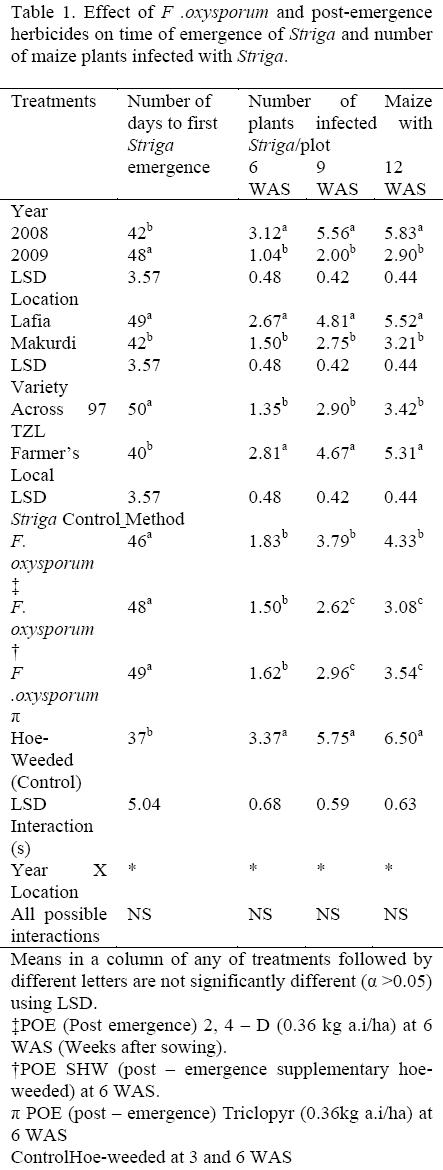

Number of maize plants infected with Striga differed significantly at 6, 9 and 12 WAS with respect to year, location, and varieties. The different Striga control methods were different only at 9 and 12 WAS (Table 1). Generally, number of maize plants infected with Striga was highest throughout the period of observation in 2008 than 2009. Similarly, number of maize plants infected with Striga was highest throughout the period of observation at Makurdi than at Lafia. The farmer's local variety recorded the highest number of maize plants infected with Striga during the same period of observation. In the Striga control methods, the hoe-weeded check recorded significantly higher number of maize plants infected with Striga throughout the period of when compared to all other Striga control treatments (Table 1). At 6 WAS, the least was obtained with plots that received F. oxysporum that was fb post-emergence (POE) application of either Triclopyr or 2 4-D at the rate of 0.36 kg a.i/ha or supplementary hoe-weeded (SHW) at 6 WAS, but at 9 and 12WAS, it was observed with plots that received F. oxysporum fb POE Triclopyr at the rate of 0.36 kg a.i/ha and SHW. This work agrees with earlier report by Ciotola et al (2000) who had provided evidence of complete inhibition of S .hermonthica emergence when a chlamydospores containing powder was added to the soil at planting or when sorghum seeds coated with chlamydospores were sown. Marley et al (2004) also revealed that treatments of plots at sowing by spot application of 5g of Fusarium sp- colonized grains in each planting hole equivalent to 165 kg/ha was found very effective in the control of S. hermonthica. There were significant interactions between year and location on number of maize plants infected with Striga at 6, 9 and 12 WAS (Table 3). The 2008 Lafia interactions throughout the period of observation recorded the highest number of maize plants infected with Striga, while the lowest was recorded by the 2009 X Lafia interactions.
Table 4, presents the result of Striga shoot count that was significantly affected at 6, 9 and 12 WAS by the different treatments. The lowest Striga shoot count was recorded in 2009 throughout the period of observation when compared to 2008. Makurdi location recorded lower Striga shoot count/4.5m2 than Lafia location. However, the farmer's local maize variety gave significantly higher Striga shoot than those of Across 97 TZL throughout the period of observation. In the context of this paper 'resistant' refers to host cultivars that are less attacked in terms of damage and number of emerged Striga plants (Parker and Riches, 1993). Across 97 TZL being a resistant variety has been reported to produce lower amounts of germination stimulants in root exudates, leading to smaller numbers of attached parasites and/or to later attachment of the parasites to the host (Gurney et al., 2000). Parker and Riches (1993) suggested three ways by which crop cultivars resist Striga attack: Mechanical barrier to the host root cells that prevent the haustoria from attaching, anti-haustoria initiation factors, and low stimulant production. In the Striga control methods, hoe-weeded check gave significantly higher Striga shoot, while at 9 and 12 WAS, the lowest occurred with F. oxysporum fb SHW and then followed by plots that received F. oxysporum fb POE application of Triclopyr or 2, 4-D each at 0.36 kg a.i/ha (Table 4). This result is in accordance with previous findings in which the isolate F. oxysporum (Foxy 2) was able to reduce the germination of S. hermonthica seeds by more than 90% when the fungus was applied during the seed- conditioning phase and it prevented the emergence by 98% when it was used as soil inoculum (Kroschel et al., 1996). This was also the case of F.nygamia attacking S. hermonthica on sorghum and pearl millet in West Africa (Abbasher, 1994). It is assumed that the reduction in seed germination and death of the germinated seeds before they attach, due to foxy 2, led to the reduced number of emerged S. hermonthica as well. Thus foxy 2 exerts its effect by destruction of the seeds and prevention of emergence and subsequent reproduction. There were significant interactions between year and locations on Striga shoot count at 6, 9 and 12 WAS (Table 5).
The 2008 X Lafia interactions throughout the period of observation recorded the highest Striga shoot count, but the 2009 X Lafia interactions recorded the lowest.
Number of Striga capsules/plant differed significantly among the various treatments, except year effect (Table 4). Although not significant, the number of Striga capsules/ plant was higher in 2009 than 2008. However, location effect produced significant difference with Makurdi recording the highest number of capsules/ plant (95) as compared to Lafia (88). Similarly, the farmer's local variety recorded the highest of 114 Striga capsules/plant as against 69 capsules observed in the cultivar Across 97 TZL. The maize cultivar Across 97 TZL had earlier been reported to be resistant to Striga (Parker and Riches, 1993).
Crop vigour at 6 and 12 WAS, differed significantly at the different treatments (Table 6). In 2009, more vigorous maize plants were observed than in 2008 throughout the period of observation (6 and 12 WAS). Although, not significant, Makurdi recorded more vigorous maize plants than at Lafia. This may be attributed to environmental differences between the two sites and the fewer number of maize plants infected with Striga at Makurdi than Lafia. Throughout the period of observation, the cultivar Across 97 TZL recorded more vigorous maize plants than the farmer's local variety. The tested maize varieties and hybrids are les damaged owing to resistance to Striga ( Kim, 1994; Berner et al., 1995 ). In the Striga control methods, at both periods off observation, the hoe-weeded check had less vigorous maize plants as compared to all plots that received F. oxysporum fb POE application of either 2,4-D, Triclopyr and SHW. There were significant interactions between year and locations on crop vigour score at 6 and 12 WAS (Table 7).Throughout the period of observation more vigorous maize plants, were recorded in the 2009 X Lafia interaction, while the less vigorous plants were observed in the 2009 X Makurdi interaction. Crop stand count at harvest was significantly affected by the different treatments except in the Striga control methods (Table 6). The result indicated significantly higher crop stand count in 2009, at Makurdi, and with cultivar Across 97 TZL. However, in the Striga control methods, although not significant, the trend indicated higher crop stand count in all plots that received F. oxysporum when compared to the hoe-weeded.

Weight of 1000 grains was only significantly different with respect to year and Striga control methods (Table 6). The weight of 1000 grains in 2008 was significantly heavier than those of 2009. Although not significantly different, Makurdi location and the farmer's local variety recorded heavier grains over Lafia and Across 97 TZL variety. This can be attributed to varietal difference. However, in the Striga control methods, the use of F. oxysporum fb POE application of either SHW or Triclopyr gave significantly heavier grains as compared to hoe-weeded check. This could be attributed to the fact that less Striga infestation/shoot count was observed in these treatments/plots with resultant less attack on maize plants and consequently better growth that gave heavier grains.
Maize grain yield was significantly influenced by maize varieties and the different Striga control methods (Table 6). Although not significantly different, the trend indicated that maize grain yield of 2009 and at Makurdi location were higher than that of 2008 and Lafia location. The low yield at Lafia may be attributed to presence of more seeds of the parasitic plant when compared to Makurdi. Among the tested maize varieties, Across 97 TZL recorded significantly higher maize grain yield than the farmer's local variety. It has earlier been reported that improved open pollinated (OP) maize varieties and hybrids are less damaged owing to tolerance to Striga and thereby produce higher grain yields than the susceptible cultivars (Berner et al., 1995; Kim et al., 1997). In the Striga control methods, the use of F. oxysporum fb either POE Triclopyr or 2, 4 – D each at 0.36 kg a.i/ha or SHW resulted in higher maize grain yields than the hoe- weeded check. This result agrees with earlier research by Lagoke et al (1997) when 2, 4 – D alone, mixture with diflufenican, Triclopyr resulted in higher grain yield than the control. The low yield in the hoe- weeded check can be attributed to higher level of Striga infestation/shoot count in those plots. The use of F. oxysporum followed by either POE application of 2, 4 – D or Triclopyr at the rate of 0.36 kg a.i/ha can be effective in suppressing the parasitic plant Striga.
CONCLUSION
Several decades of research on Striga control technologies have resulted in the identification of a range of technologies. Farmers themselves have developed a range of coping strategies to combat Striga. As none of the available technologies on its own can provide satisfactory Striga control in broad range of biophysical and socio-economic environments, many farmers in West Africa fail to control Striga in cereals, despite the availability of a whole range of control techniques that have proved to be successful. Therefore, there is the need to look at other control options like the use of mycoherbicides that will be environmentally friendly. This study results demonstrate the high potentiality of using F. oxysporum for the control of S. hermonthica as pre-plant (spot application) and there after followed by POE application of either 2, 4 – D or Triclopyr at 6 WAS. The use of maize grits, which is readily available to propagate F. oxysporum makes it quite cheap for local farmer's instead of the use of potato dextrose agar.
REFERENCES
Abbasher, A.A. 1994. Microorganisms associated with Striga hermonthica and their possibilities of their utilization of biological control agents. PLITS, 12, Weikersheim, Germany: MargrafVerlag [ Links ]
Abbasher, A.A.; Kroschel, J.; Sauerborn, J. 1995. Micro-organisms Striga hermonthica in Northern Ghana with potential as biocontrol agents. Biocontrol Science and Technology, 5: 157-161 [ Links ]
Abbasher, A.A.; Hess, D.E.; Sauerbon, J. 1998. Fungal pathogens for biological control of Striga hermonthica on sorghum and pearl millet in West Africa. African Crop Science Journal, 6: 179-188 [ Links ]
Berner, D.K.; Kling, J.G.; Singh, B.B. 1995. Striga Research and control: A perspective from Africa. Plant diseases, 79: 652 – 670 [ Links ]
Carson, A. 1988. Development and testing of a control package of Striga hermonthica on small-scale holdings in the Gambia. Tropical Pest Management, 34: 97 – 101. [ Links ]
Ciotola, M.; Watson, A.K.; Hallet, S.G. 1995. Discovery of an Isolate of Fusarium oxysporum with potential to control Striga hermonthica in Africa. Weed Research, 35: 303-309. [ Links ]
Ciotola, M.; Ditommaso, A.; Watson, A.K. 2000. Chlamydospores production, inoculation methods and Pathogenicity of Fusarium oxysporum M12 – 4A, a biocontrol fork Striga hermonthica. Biocontrol Science and Technology, 10: 129-145 [ Links ]
Emechebe, A.M.; Lagoke, S.T.O.; Adu, J.K. 1991. Research Towards Integrated control of Striga in West and Central Africa, in Progress in Food Grain Research, (eds.). J. M. Menyonga, T. Bazuneh, J.Y. Yayock and I. Soumana, Burkina Faso: (OAU/STRC-SAFGRAD), pp. 445-463. [ Links ]
Emechebe, A.M.; Ellis-Jones, J.; Schulz, S.; Chikoye, D.; Douthwaite, B.; Kureh, I.; Tarawali, G.; Hussaini, A.M.; Kormawa, P.; Sanni, A. 2004. Farmers perception of the Striga problem and its control in Northern Nigeria. Experimental Agriculture, 40: 215- 232 [ Links ]
Frost, D.L.; Gurney, A.L.; Press, M.C.; Scholes, J.D. 1997 .Striga hermonthica reduces photosynthesis in sorghum: the importance of stomatal limitations and a potential role of ABA?. Plant cell and Environment, 20: 483-492 [ Links ]
Gurney, A.L.; Press, M.C.; Ransom, J.K. 1995. The parasitic angiosperm Striga hermonthica can reduce photosynthesis of its sorghum and maize host in the field. Journal of Experimental Botany, 46: 1817-1823 [ Links ]
Gurney, A.L.; Taylor, A.; Mbwaga, A.; Scholes, J.D.; Press, M.C. 2002. Do maize cultivars demonstrate tolerance to the parasitic weed Striga asiatica? Weed Research, 42: 299-306. [ Links ]
Johnson, D.; Riches, C.; Diallo, R.; Jones, M. 1997. Striga on rice in West Africa; crop host range and the potential of host resistance. Crop Protection, 16: 153- 520 [ Links ]
Kim, S.K. 1994. Genetics of maize tolerance to Striga hermonthica. Crop Science, 34:900- 907. [ Links ]
Kim, S.; Adetimirin, V.O.; Akintunde, A.Y. 1997. Nitrogen effects on Striga hermonthica infestation, grain yield and agronomic traits of tolerant and susceptible maize hybrids. Crop Science, 37: 711- 716. [ Links ]
Kroschel, J.; Hundt, A.; Abbasher, A.A.; Sauerborn, J. 1996. Pathogenicity of fungi collected in Northern Ghana to Striga hermonthica. Weed Research, 36: 515- 520. [ Links ]
Lagoke, S.T.O.; Shebayan, J,Y.; Magani, I.E.; Oluronjo, P.; Olufajo, O.O.; Elemo, K.A.; Uvah. I.; Adeoti; A.A.; Chindo, P.S.; Kureh, I.; Jatau, S.; Emechebe, A.M.; Ndahi, W.B.; Kim, S.K.; Webber, G.; Singh, B.B.; Odion, C.; Avav, T. 1997. Striga problems and development of appropriate control technologies in various crops in Nigeria, in Intergrated Management of Striga for the African Farmer, (eds.). S. T.O Lagoke E.I. Vander Straten and S.S. M' Boob, Proceedings of 3rd General Workshop of Pan African Striga control Network (PANSCON) 18- 23rd October, Harare, Zimbabwe, 157 pp. Accra, (Ghana) FAO. [ Links ]
Marley, P.S.; Ahmed, S.M.; Shebayan, J.A.; Lagoke, S.T.O. 1999. Isolation of Fusarium oxysporum with potential for biocontrol of the witch weed Striga hermonthica in the Nigerian Savanna. Biocontrol Science and Technology, 9: 159-163. [ Links ]
Marley, P.S.; Toure, A.; Shebayan, J.A.; Aba, D.A.; Toure, O.A.; Diallo, G.A.; Katile, S.O. 2004. Variability in host plant resistance of Sorghum to Striga hermonthica in West Africa. Archives of Phytopathology and plant protection, 37: 29-34. [ Links ]
Marley, P.S.; Kroschel, J.; Elzein, A. 2005. Host specificity of Fusarium oxysporum Schlect (isolate PSM 197), a potential mycoherbicide for controlling Striga spp. in West Africa. Weed Research, 45: 407-412. [ Links ]
Parker, C. 1991. Protection of crops against parasitic weeds. Crop Protection, 10: 6-22. [ Links ]
Parker, C.; Riches, C.R. 1993. Parasitic Weeds of the World: Biology and Control, Wallingford, Oxon, UK: CAB International, 304 pp. [ Links ]
Stewart, G. 1990. Witch weed: a parasitic weed of grain crops. Outlook on Agriculture, 19, 115-117. [ Links ]
Thalouaran, T.A.; Fer, A. 1993. Le Striga, un revageur de cultures vivrieres: le point sur les connaissances recentes et sur les methods de lutte. Cahiers Agricultures, 2: 167-182 [ Links ]














